44 chemical equation labels
Answered: Please convert each word equation to a… | bartleby Science Chemistry Please convert each word equation to a chemical equation (including all phase labels) • Reaction 3: Copper (II) hydroxide decomposes upon exposure to heat to yield cupric oxide and water. Cupric oxide is a black solid. How to balance chemical equations? Chemistry Q&A - Byju's
How would you label each formula in the chemical equation below ... Jan 23, 2017 ... The reactants are on the LEFT HAND SIDE of the equation. The products are on the right hand side. So for the oxidation of iron,. Fe+S→FeS.
Chemical equation labels
opentextbc.ca › introductorychemistry › chapterThe Chemical Equation – Introductory Chemistry – 1st Canadian... Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as temperature, may also be listed above the arrow. For example: 2NaHCO 3 (s) → 200°C Na 2 CO 3 (s) + CO 2 (g) + H 2 O (ℓ) Key Takeaways Balancing Chemical Equations | CK-12 Foundation 4.1: Writing and Balancing Chemical Equations Begin by identifying formulas for the reactants and products and arranging them properly in chemical equation form: CO 2(aq) + NaOH(aq) → Na 2CO 3(aq) + H 2O(l) Balance is achieved easily in this case by changing the coefficient for NaOH to 2, resulting in the molecular equation for this reaction: CO 2(aq) + 2 NaOH(aq) → Na 2CO 3(aq) + H 2O(l)
Chemical equation labels. 7.3: Chemical Equations - Chemistry LibreTexts Use the common symbols, ( s), ( l), ( g), ( a q), and → appropriately when writing a chemical reaction. In a chemical change, new substances are formed. In order for this to occur, the chemical bonds of the substances break, and the atoms that compose them separate and rearrange themselves into new substances with new chemical bonds. 7.3: The Chemical Equation - Chemistry LibreTexts Jul 18, 2022 ... Reactants and Products ... To describe a chemical reaction, we need to indicate what substances are present at the beginning and what substances ... Answered: Please convert each word equation to a… | bartleby Science Chemistry Please convert each word equation to a chemical equation (including all phase labels) Reaction 5: Cupric sulfate reacts with elemental aluminum to create aluminum sulfate and elemental copper. Cupric sulfate dissolves in water to produce a blue solution due to the formation of the hydrated copper (II) cation. Solved q20.) Write the chemical equation that represents the | Chegg.com Science. Chemistry. Chemistry questions and answers. q20.) Write the chemical equation that represents the dissolving of solid Mg (NO3)2 in water. Include all phase labels. (Example format XX− (aq)+XX3+ (aq) ) Mg (NO3)2 (s) →. Question: q20.) Write the chemical equation that represents the dissolving of solid Mg (NO3)2 in water.
quizlet.com › 701655616 › chem-chapter-5-smartbooks-flash-cardsCHEM Chapter 5 SmartBooks Flashcards | Quizlet Place the following steps for writing and balancing a chemical equation in order. The first step should be at the top of the list. 1. write the correct formulas for reactants and products, separated by an arrow 2. add the physical state symbol for all substances 3. count the atoms of each element on the reactant side and on the product side en.wikipedia.org › wiki › Chemical_equationChemical equation - Wikipedia A chemical equation is the symbolic representation of a chemical reaction in the form of symbols and chemical formulas. The reactant entities are given on the left-hand side and the product entities are on the right-hand side with a plus sign between the entities in both the reactants and the products, and an arrow that points towards the ... Chemical Equation - Chemical Reaction and equation, Class 10 For the chemical equations shown below, label each reactant as ... Answer to: For the chemical equations shown below, label each reactant as either acid or base, and each product as either conjugate acid or...
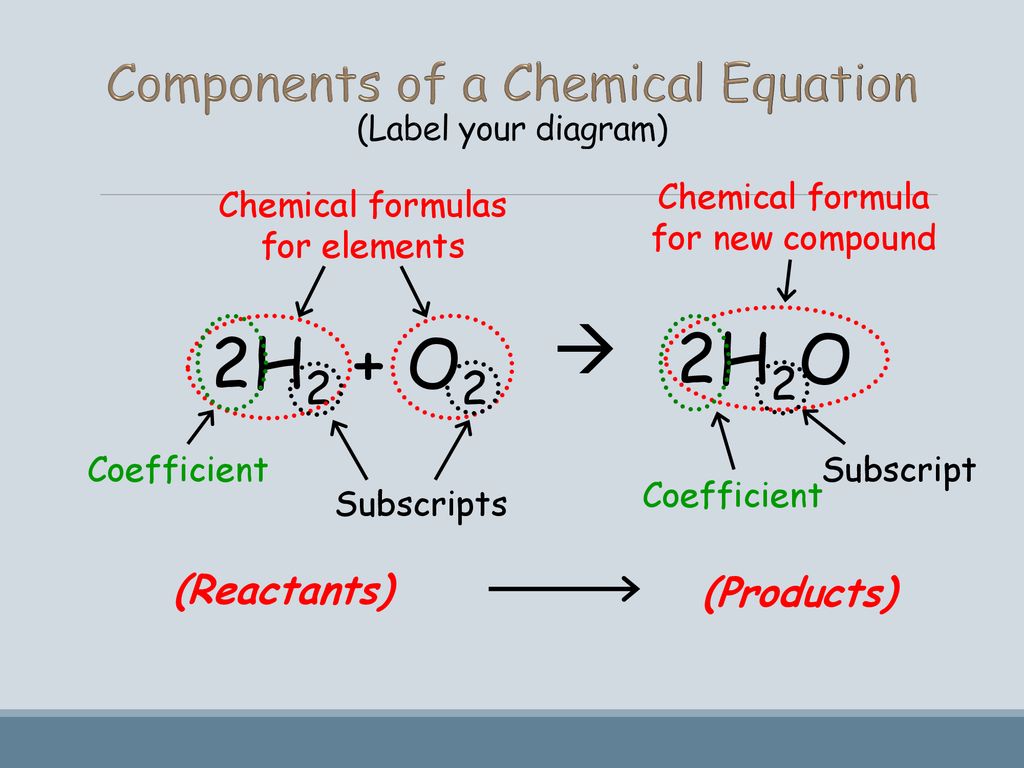
What are the Parts of a Chemical Equation? | Life Persona 1- Let's start by simply writing a chemical equation in terms of the formulas of substances, remembering that both elemental hydrogen and chlorine are diatomic: H 2 + Cl 2 → HCl There are two hydrogen atoms and two chlorine atoms in the reactants and one of each atom in the product. Laebling A Chemical Equation Part I - YouTube Aug 5, 2012 ... Labeling chemical equations and identifing reactants, yield and products. 2.4 Flashcards | Quizlet Label the reactants and products in the chemical reaction: CH4+2O2----------------------> CO2+2H2O. Write brief definitions for these terms next to their labels. CH4+2O2- reactants: the substances changed during a chemical reaction; they are located on the left side of the equation Labeling A Chemical Equation Part 2 - YouTube Aug 5, 2012 ... Calculating Acceleration · How to Use the Activity Series · SatoxCoin | Best Token in 2023 - How To Buy it · Balancing Chemical Equations Practice ...
Chemical formula - Wikipedia Chemical formula. In chemistry, a chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as parentheses, dashes, brackets, commas and plus (+) and minus (−) signs.
Balancing Chemical Equations: Practice and Review - Albert.io
7.4: How to Write Balanced Chemical Equations Count the numbers of atoms of each kind on both sides of the equation to be sure that the chemical equation is balanced. Example 7.4.1: Combustion of Heptane. Balance the chemical equation for the combustion of Heptane ( C 7H 16 ). C 7H 16(l) + O 2(g) → CO 2(g) + H 2O(g)
Symbols in Chemical Equations - Harper College Symbols in Chemical Equations ; Symbol, Meaning ; +, used to separate one reactant or product from another ; used to separate the reactants from the products - it ...
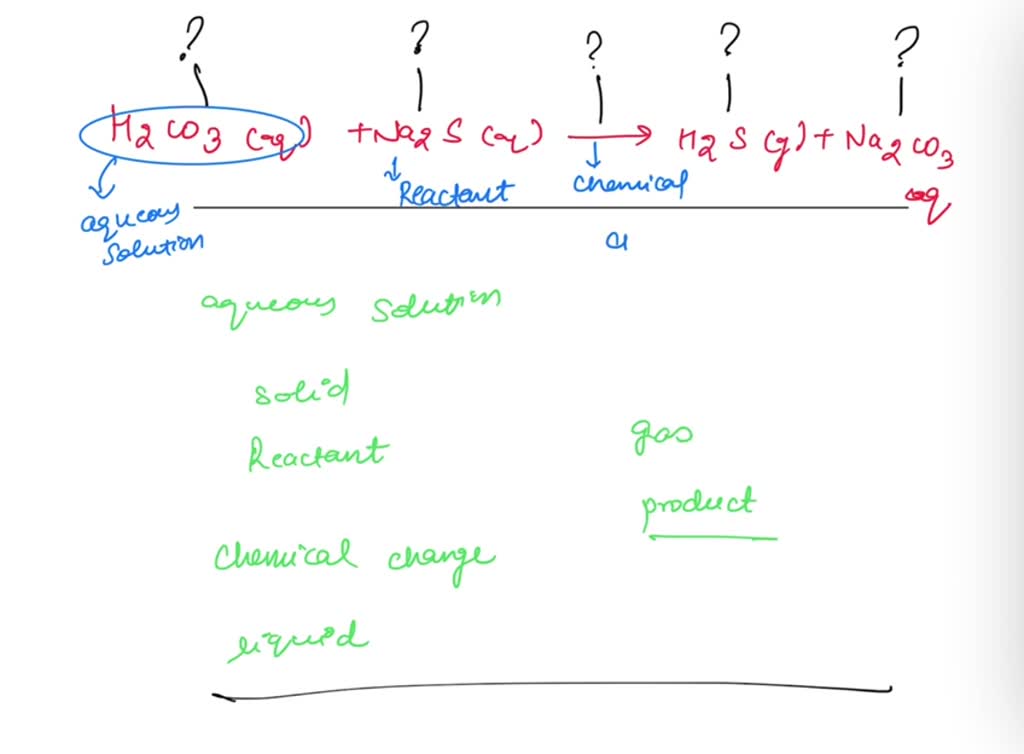
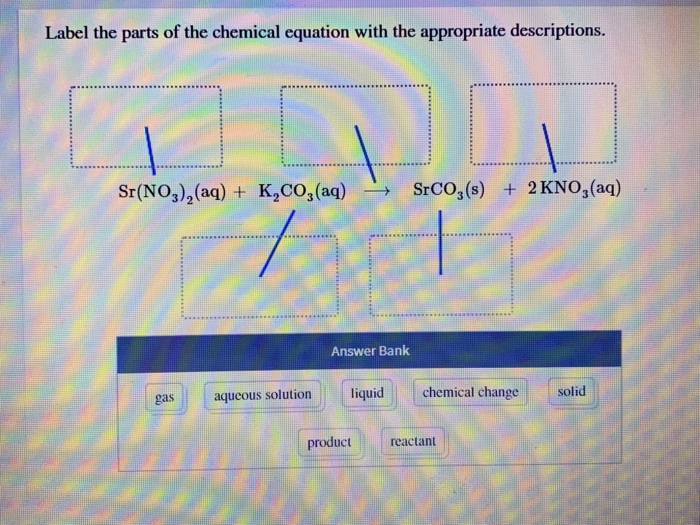
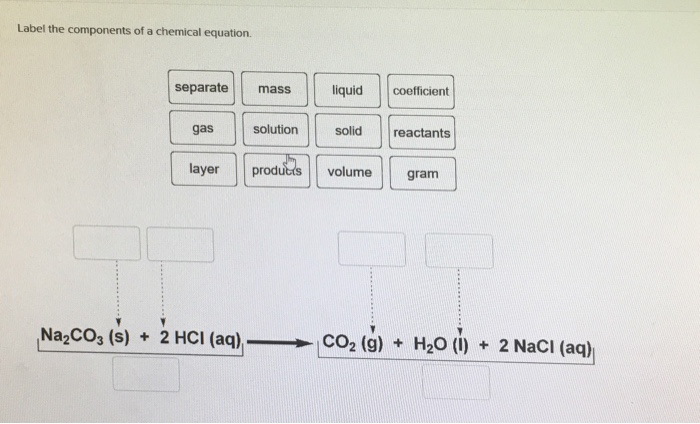
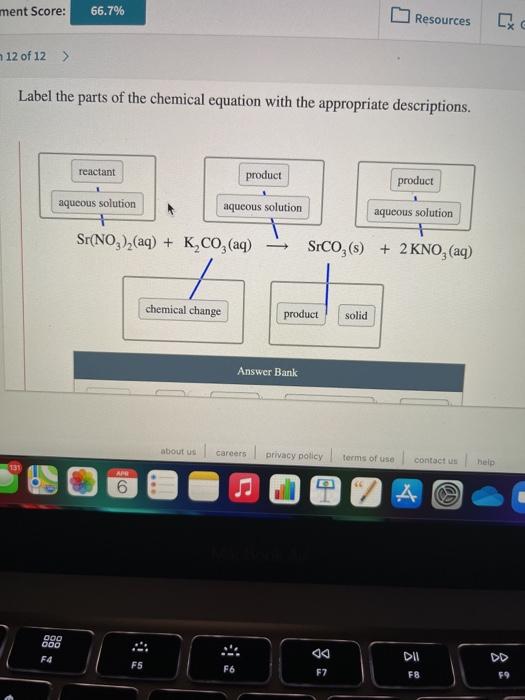
Label the parts of the chemical equation with the appropriate descriptions, HzCOz(aq), Naz S(aq), HzS(g), Naz COz(aq), Answer Bank, aqucous solution, solid, reactant, chemical change, liquid, product
Align and label in chemical equation - TeX - LaTeX Stack Exchange Apr 17, 2017 ... Align and label in chemical equation · How to only label the second, third and sixth line with 1, 2, 3? · the arrow with catalyst in first line is ...
Chemical Equation Balancer Practice by balancing a few of the equations below. If you get stuck, click the links to use our chemical equation balance calculator to see the balanced result and the four easy steps to get there: Aluminium + Sodium Hydroxide + Water = Sodium Aluminate + Hydrogen Gas: Al + NaOH + H2O = NaAlO2 + H2.
study.com › academy › lessonWhat is a Chemical Equation? - Definition & Examples Oct 28, 2021 · Chemical equations tell us the elements and/or compounds that are reacting and what the product (s) of the reaction. The coefficients on the substances in the reaction tell us the mole ratio or...
How to Write a Chemical Equation (with Pictures) - wikiHow All chemical equations look something like "A + B →C (+ D...)," in which each letter variable is an element or a molecule (a collection of atoms held together by chemical bonds). The arrow represents the reaction or change taking place.
How do you Write a Chemical Equation? - A Plus Topper A chemical equation is a shorthand representation of a chemical reaction using the symbols and formulae of substance involved in the chemical reaction. The symbols and formulae of the substances (elements or compounds) are arranged to show the reactants and products of a chemical reaction.
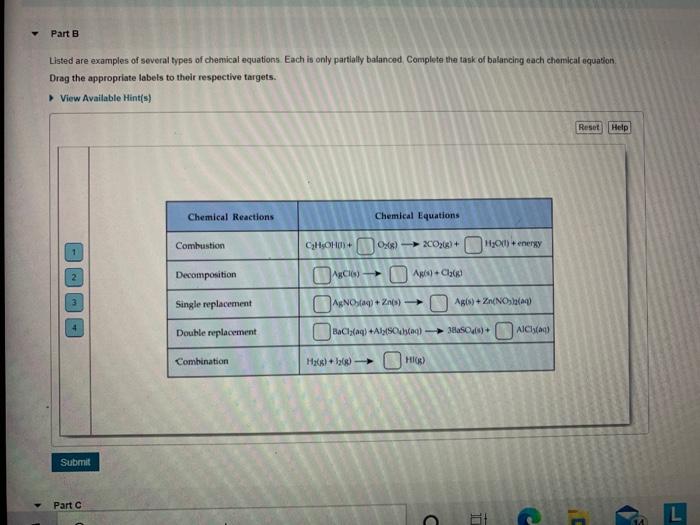
Balancing Different Types of Chemical Equations - Quizlet HW5-Chapter5-Chem106 - Balancing Different Types of Chemical Equations. 5.0 (2 reviews) Term. 1 / 3. Balance the chemical equation by indicating the number of each species in the appropriate blanks. For this exercise, indicate coefficients of 1 explicitly.
Label each reactant and product in the given chemical reaction. Write the products for the given sequence of the reaction. Predict the product or reactant in the depicted reaction. If there is more than one product, circle ...
3.1: Chemical Equations - Chemistry LibreTexts The initial (unbalanced) equation is as follows: Ca 5(PO 4) 3(OH)(s) + H 3PO 4(aq) + H 2O ( l) → Ca(H 2PO 4) 2 ⋅ H 2O ( s) 1. B Identify the most complex substance. We start by assuming that only one molecule or formula unit of the most complex substance, Ca 5(PO 4) 3(OH), appears in the balanced chemical equation. 2. Adjust the coefficients.
byjus.com › chemistry › chemical-equationWhat are Chemical Equations? Detailed Explanation, Examples -... Chemical equations were first formulated by the French chemist Jean Beguin in the year 1615. Chemical reactions can be represented on paper with the help of chemical equations, an example for which is represented below (for the reaction between hydrogen gas and oxygen gas to form water). 2H 2 + O 2 → 2H 2 O
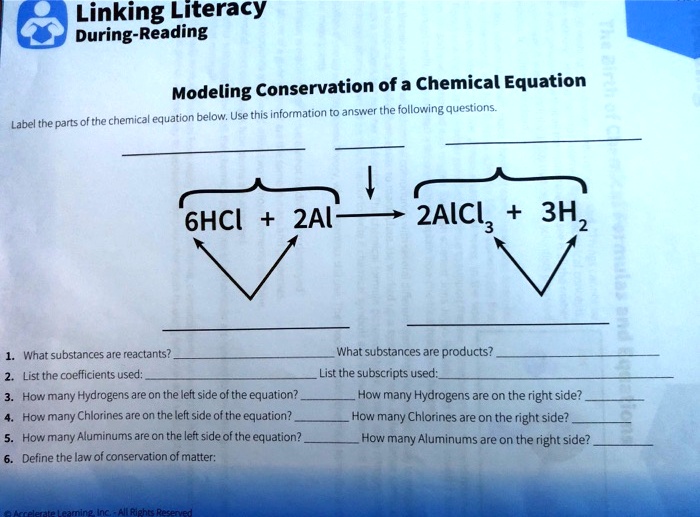
Chapter 4 Quantities of reactants and products Phase labels: letters written in parenthesis after a reactant or product to indicate whether the substance is a solid (s), liquid (l), gas (g) or dissolved in water (aq). Stoichiometric coefficients: number placed in front of formulas in a chemical equation to balance the numbers of each type of atom in the entire chemical equation.
› Browse › Search:chemical formula labelsChemical Formula Labels Teaching Resources | Teachers Pay... Use this digital interactive notebook to teach your students about chemical formulas and chemical reactions.The journal introduces and walks students through how to count the atoms.Then they break down an equation with drag and drop labels.The students end with vocabulary and scenarios.
4.E: Chemical Reactions and Equations (Exercises) Write a chemical reaction for the boiling of water, including the proper phase labels. Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why. 4Na(s) + 2Cl 2 (g) → 4NaCl(s) should not be considered a proper chemical equation ...
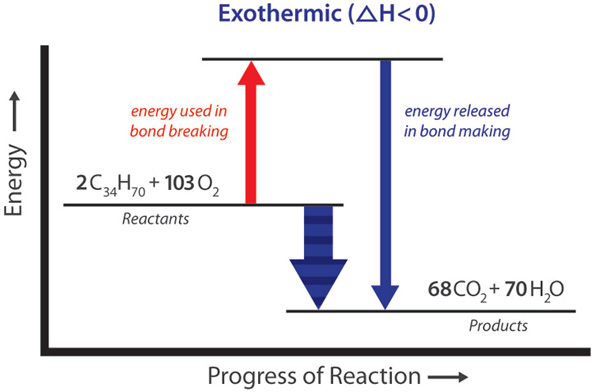
› examples-of-10-balanced-chemical-equations-604027Examples of Balanced Chemical Equations - ThoughtCo Oct 2, 2019 · When you balance a chemical equation, it's always a good idea to check the final equation to make sure it works out. Perform the following check: Add up the numbers of each type of atom. The total number of atoms in a balanced equation will be the same on both sides of the equation.
4.1: Writing and Balancing Chemical Equations Begin by identifying formulas for the reactants and products and arranging them properly in chemical equation form: CO 2(aq) + NaOH(aq) → Na 2CO 3(aq) + H 2O(l) Balance is achieved easily in this case by changing the coefficient for NaOH to 2, resulting in the molecular equation for this reaction: CO 2(aq) + 2 NaOH(aq) → Na 2CO 3(aq) + H 2O(l)
Balancing Chemical Equations | CK-12 Foundation
opentextbc.ca › introductorychemistry › chapterThe Chemical Equation – Introductory Chemistry – 1st Canadian... Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as temperature, may also be listed above the arrow. For example: 2NaHCO 3 (s) → 200°C Na 2 CO 3 (s) + CO 2 (g) + H 2 O (ℓ) Key Takeaways


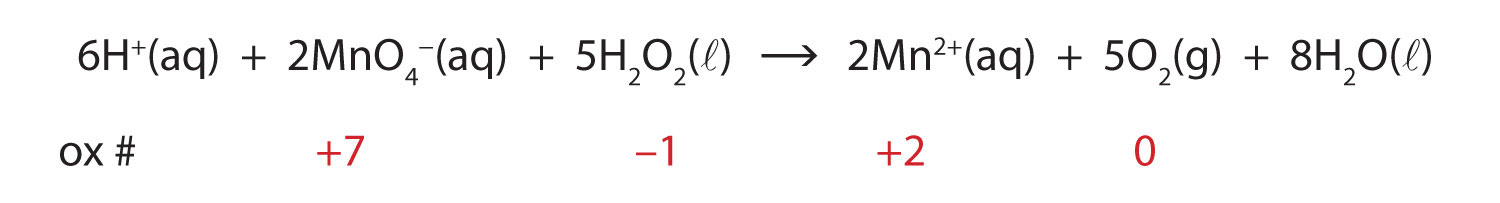






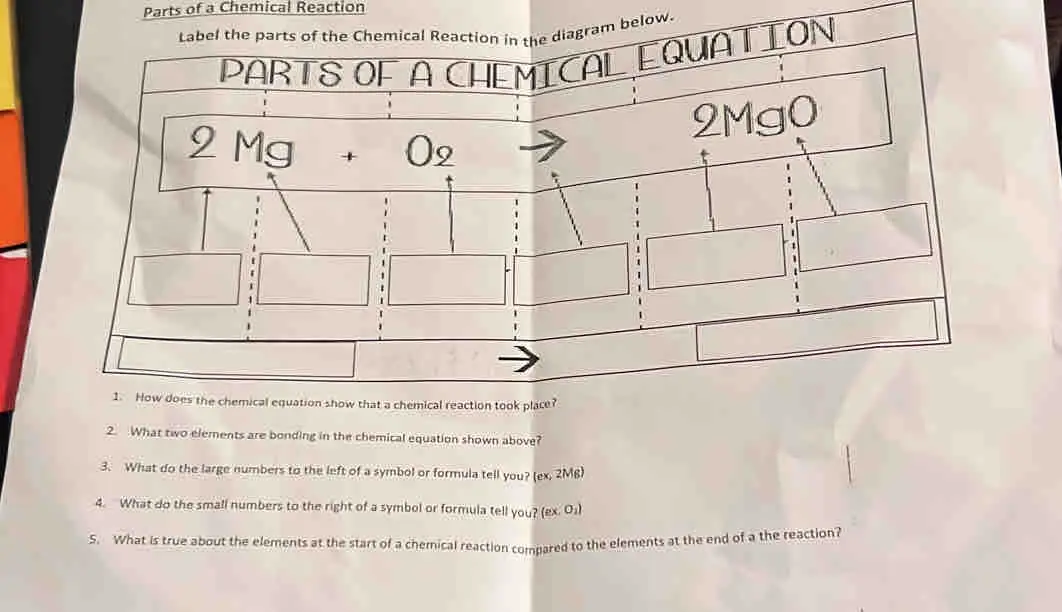








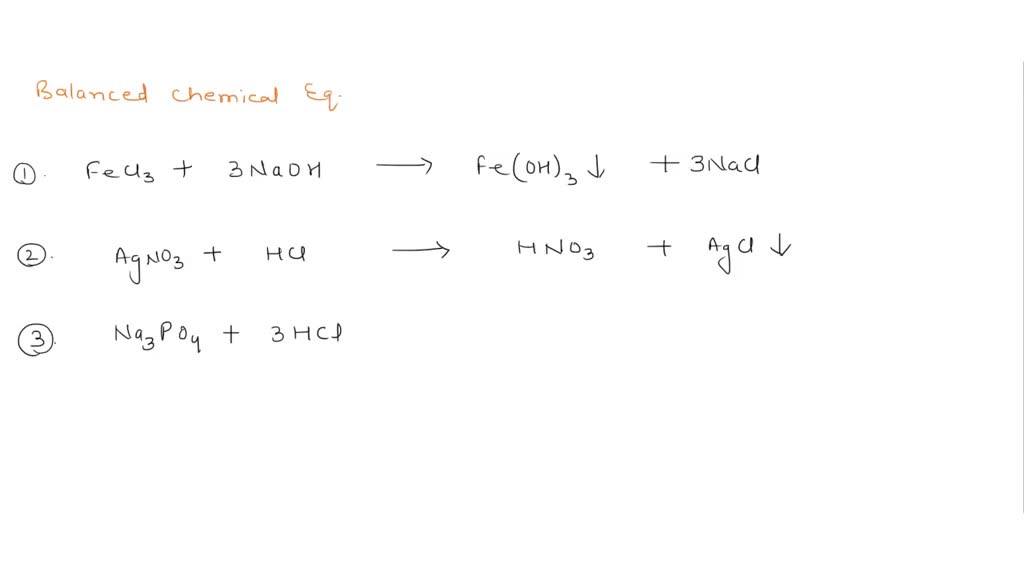







Post a Comment for "44 chemical equation labels"