45 open label extension study
NLS Pharmaceutics Announces Open Label Extension Study Six-Month Data ... "The open label extension study further validates the safety and tolerability seen in the DB Phase 2 trial, while demonstrating further efficacy gains with longer treatment duration, beyond the ... Open-label trial - Wikipedia Open-label trials may be appropriate for comparing two similar treatments to determine which is most effective, such as a comparison of different prescription anticoagulants, [4] or possible relief from symptoms of some disorders when a placebo is given. [5] An open-label trial may still be randomized.
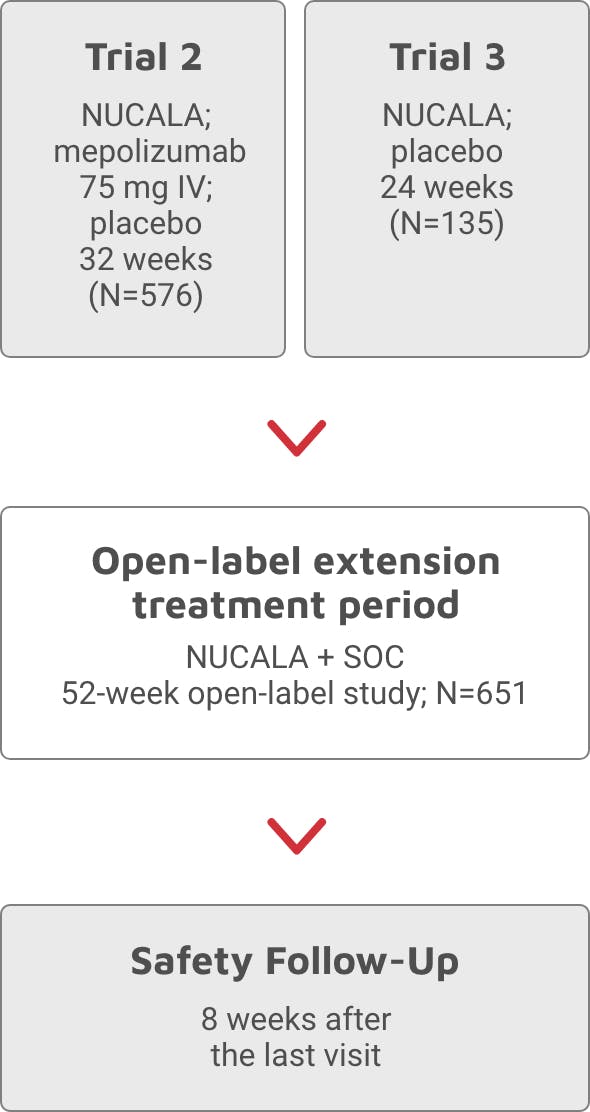
PDF Core and extension studies final - PharmaSUG Core and Extension studies are sometimes referred to as main study and open label Extension studies. Most of the time main studies follow double blind procedures to randomize subjects and subjects can get either Placebo or study drug based on randomization. Subjects allowed to enroll into the study typically
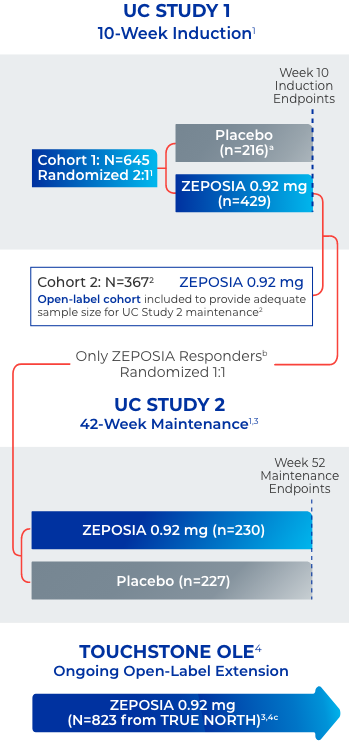
Open label extension study
Study to Assess the Effect of Long-term Treatment With Voxelotor in ... This open label extension (OLE), multi-center study will be conducted at approximately 100 clinical sites globally and will be available to eligible participants from study GBT440-031. The study will enroll participants from GBT440-031 (approximately 435) under any of the following conditions: A phase II, open-label, extension study of long-term patisiran ... In this 24-month Phase II open-label extension study, we evaluated the effects of patisiran treatment (0.3 mg/kg intravenously every 3 weeks) on safety, serum transthyretin levels, and clinical parameters. Efficacy assessments included modified Neuropathy Impairment Score +7 (mNIS+7) and multiple disease-relevant measures. ... NLS Pharmaceutics Announces Open Label Extension Study Six-Month Data ... Patients treated with Mazindol ER in the randomized Phase 2 trial showed continued improvement after rolling over into the open label extension (OLE) study...
Open label extension study. Understanding Clinical Trial Terminology: What is an Open Label ... Open-label trials can be used to compare treatments or gather additional information about the long-term effects in the intended patient population. In some instances, patients who complete one clinical trial may be eligible to continue in an open-label extension study where all participants are eligible to receive active treatment for an ... Open-Label Extension Studies | SpringerLink The open-label extension study is identified formally as a study. Depending on the jurisdiction (s) in which the study will be performed, approval will be required, at minimum, from a research ethics committee, and the study must be registered with a regulatory agency. NLS Pharmaceutics Announces Open Label Extension Study Six-Month Data ... NLS Pharmaceutics Announces Open Label Extension Study Six-Month Data for Quilience (R) (Mazindol ER) in the Treatment of Narcolepsy Type 1 and Type 2 NLS Pharmaceutics AG March 27, 2023, 8:30 AM... PDF What Are Open-Label Extension Studies For? - The Journal of Rheumatology of prolonged open-label extension. For example, the study of prednisolone remained randomized for 2 years2. The safety issues do not constitute a sufficient reason for con-ducting open-label extension studies. The third purpose may be to demonstrate continued effi-cacy of the drug over a longer period of time or to show that
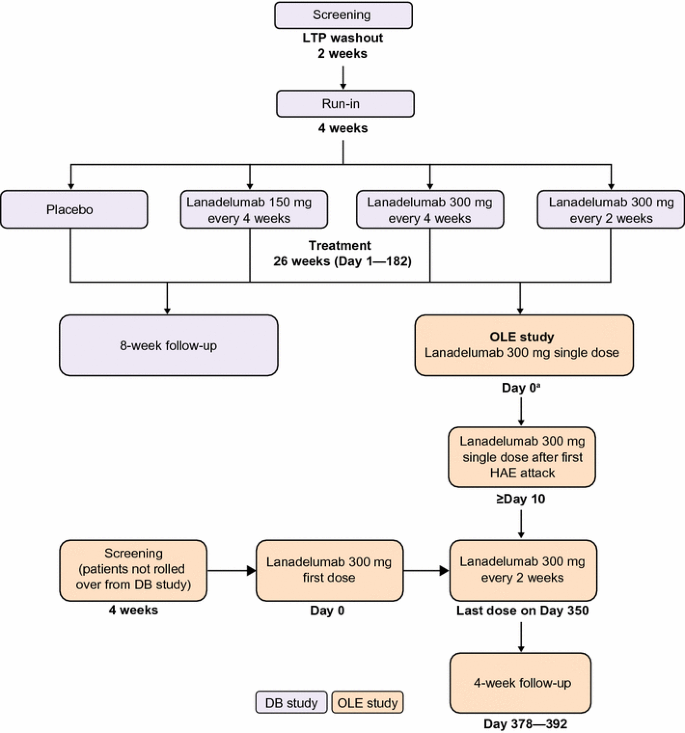
Open-label extension studies: do they provide meaningful ... - PubMed If undertaken primarily to gather more patient-years of exposure to the new drug in order to understand and gain confidence in its safety profile, open-label extension studies can play a useful and legitimate role in drug development and therapeutics. What is an open label extension study? • NCK Pharma Open-label trials may be appropriate for comparing two very similar treatments to determine which is most effective. An open-label trial may be unavoidable under some circumstances, such as comparing the effectiveness of a medication to intensive physical therapy sessions. admin Consent to open label extension studies: some ethical issues A frequent feature of pharmaceutical research is the open label extension study, in which patients participating in double blind placebo controlled trials of new medications are invited, on completion of the initial trial, to take the study drug for some further period. Patients are openly given the active substance at this stage, regardless of their assignment in the initial trial. A Study to Evaluate Rozanolixizumab in Study Participants With ... The purpose of this study is to assess the safety, tolerability and efficacy of additional 6-week treatment cycles with rozanolixizumab in study participants with generalized myasthenia gravis (gMG). Study Design Go to Resource links provided by the National Library of Medicine MedlinePlus Genetics related topics: Myasthenia gravis
Amgen Announces Results From Two Open Label Extension Studies of ... Detailed study results will be shared with regulatory authorities and submitted for presentation at an upcoming medical congress later this year. Prolonged LDL-C reduction with Repatha is also being studied in the ongoing VESALIUS-CV (NCT03872401) outcomes trial. Repatha ® Cardiovascular Open-Label Extension (FOURIER-OLE) Study Design ... New ALS Drug: What You Need To Know > News > Yale Medicine In an open-label extension study (in which patients who participated in the clinical trial were invited to keep taking the drug and be studied), 90 patients took the medication for seven more months and had a median of 4.8 months more time before being hospitalized, put on a ventilator, or dying, Amylyx reported. ... Safety and effectiveness of ulotaront (SEP-363856) in ... - Nature The aim of this 26-week open-label extension study was to evaluate the safety and effectiveness of ulotaront (25/50/75 mg/d) in patients who completed the initial 4-week study. Of the 193 4-week ... Understanding Clinical Trial Terminology: What is a Long-Term Extension ... An OLE study is a clinical trial that typically enrolls participants of a previous clinical trial and is designed to gather the long-term safety and tolerability data on a potential new medicine beyond the time period of the main study.
End of Trial and Open-Label Extension (OLE) Frequently Asked Questions ... End of Trial and Open-Label Extension (OLE) Frequently Asked Questions (FAQ) | The Dominantly Inherited Alzheimer Network End of Trial and Open-Label Extension (OLE) Frequently Asked Questions (FAQ) When did the solanezumab ("sola") and gantenerumab ("gant") blinded drug arms in the DIAN-TU trial officially end?
Open-Label Extension Study for Alzheimer's Treatment Showed Disease ... FOSTER CITY, Calif., March 28, 2023 /PRNewswire/ -- TrueBinding, Inc.— a clinical-stage biotherapeutic company creating new and exciting molecules for applications in neurodegenerative and other disease areas with great unmet needs— today announced early results from an Open-Label Extension (OLE) study for the use of its TB006 drug in the treatment of Alzheimer's Disease.
Open-Label Extension of Lecanemab Supports Safety in Early Alzheimer's The core phase IIb study had a 9.9% incidence of ARIA-E in 609 treated patients versus 7.8% of 180 participants in the open-label extension, in which all participants were treated with 10 mg/kg intravenous infusions every two weeks. Of those in the extension study who had been on placebo in the phase IIb trial, 8.9% had ARIA-E.
What is an Open-Label Clinical Trial? - News-Medical.net Open-label trials are used to compare different treatments or gather further information on the long-term effects of new drugs and treatments. In some instances, patients are eligible to...
NLS Pharmaceutics Announces Open Label Extension Study Six-Month Data ... Patients treated with Mazindol ER in the randomized Phase 2 trial showed continued improvement after rolling over into the open label extension (OLE) study...
A phase II, open-label, extension study of long-term patisiran ... In this 24-month Phase II open-label extension study, we evaluated the effects of patisiran treatment (0.3 mg/kg intravenously every 3 weeks) on safety, serum transthyretin levels, and clinical parameters. Efficacy assessments included modified Neuropathy Impairment Score +7 (mNIS+7) and multiple disease-relevant measures. ...
Study to Assess the Effect of Long-term Treatment With Voxelotor in ... This open label extension (OLE), multi-center study will be conducted at approximately 100 clinical sites globally and will be available to eligible participants from study GBT440-031. The study will enroll participants from GBT440-031 (approximately 435) under any of the following conditions:
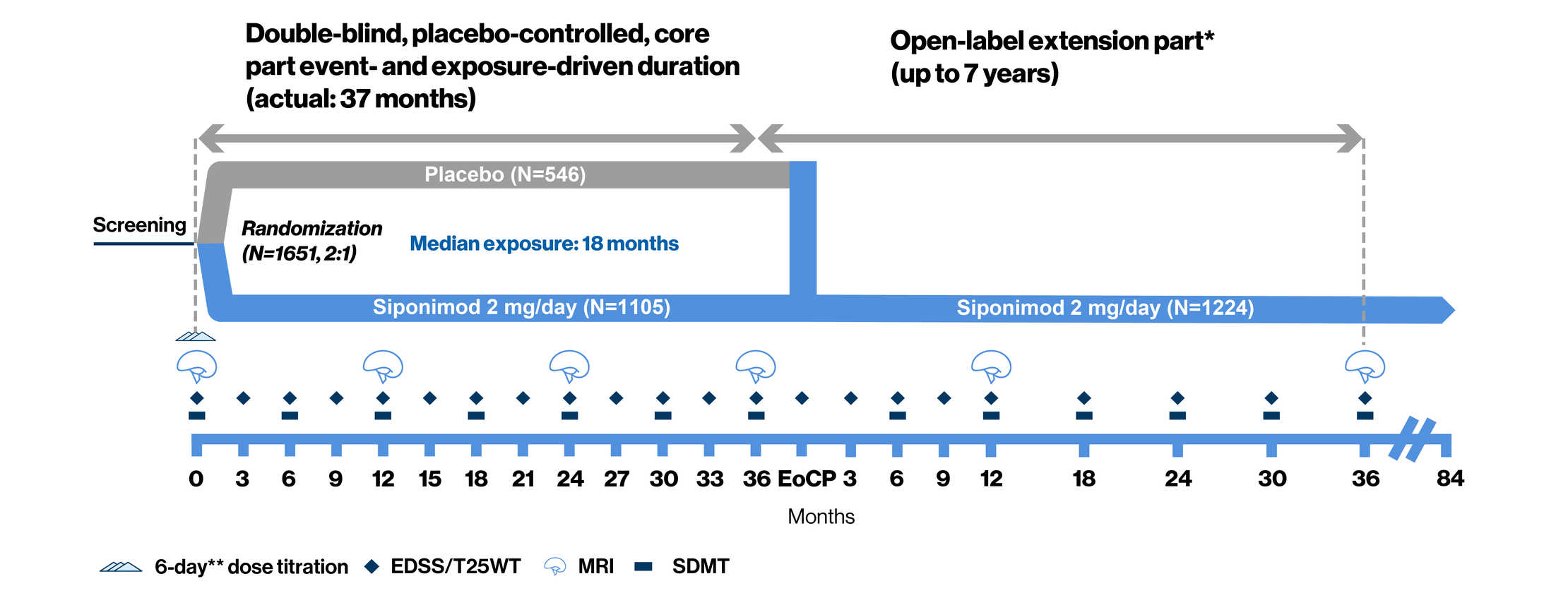








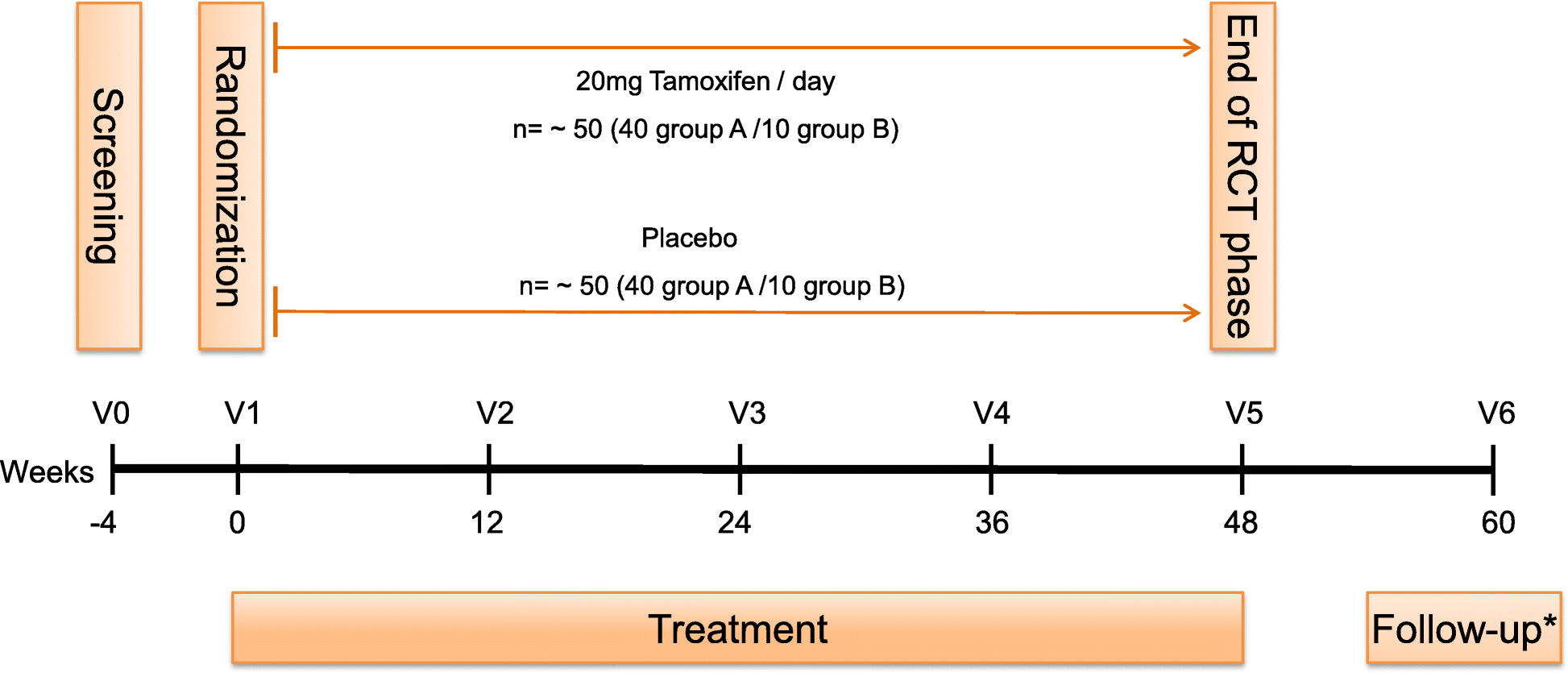























Post a Comment for "45 open label extension study"