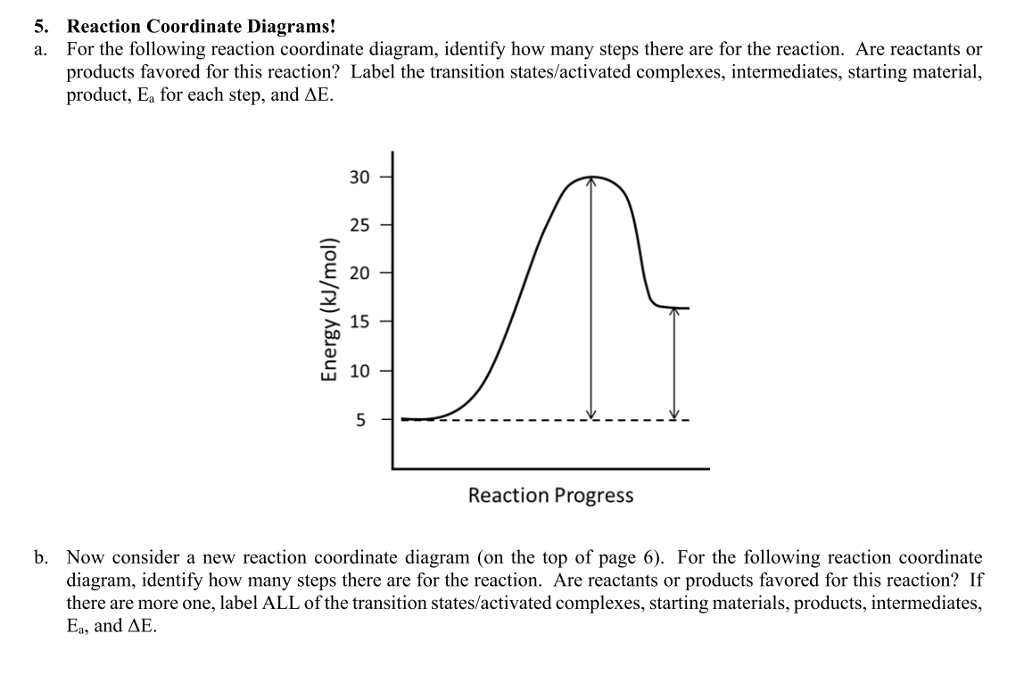
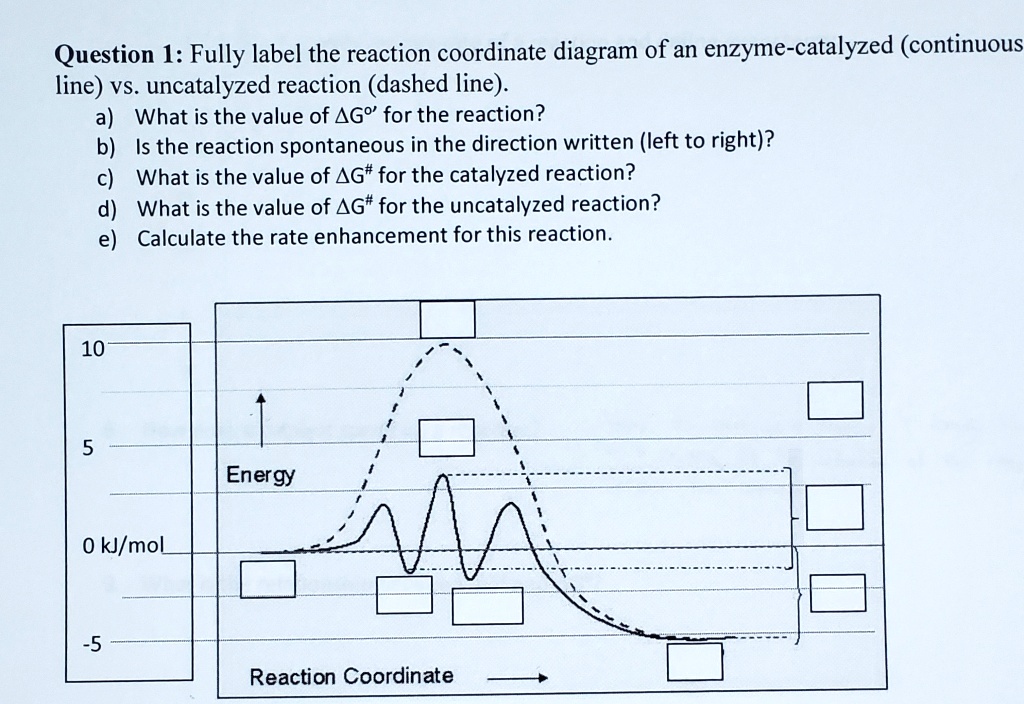
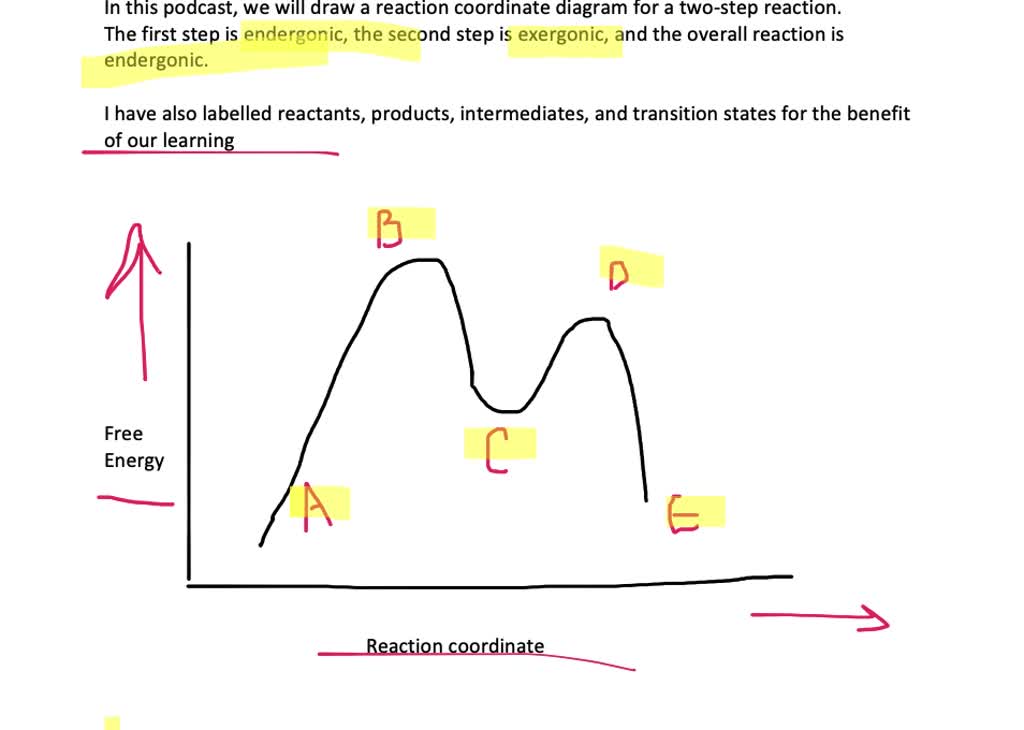
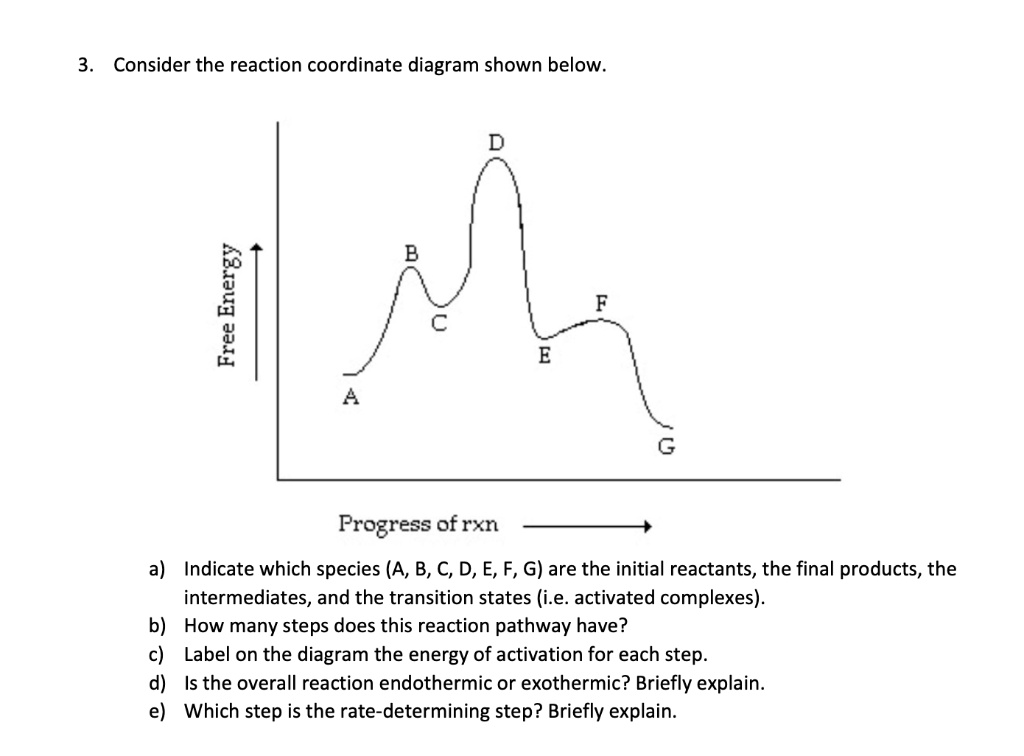
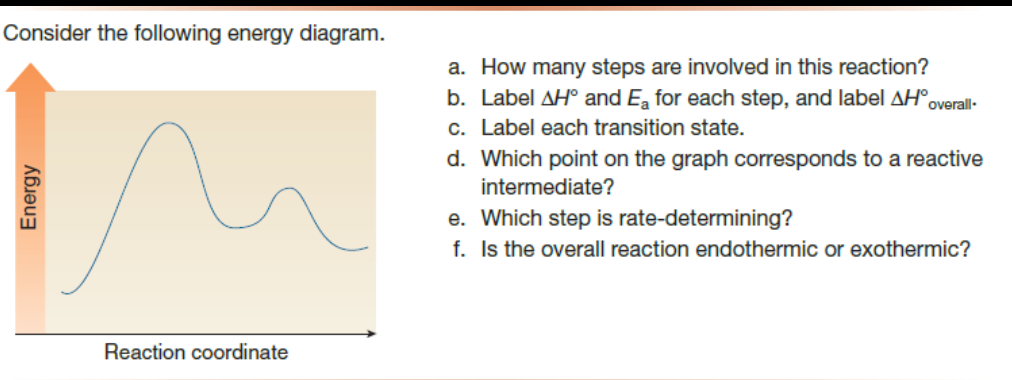
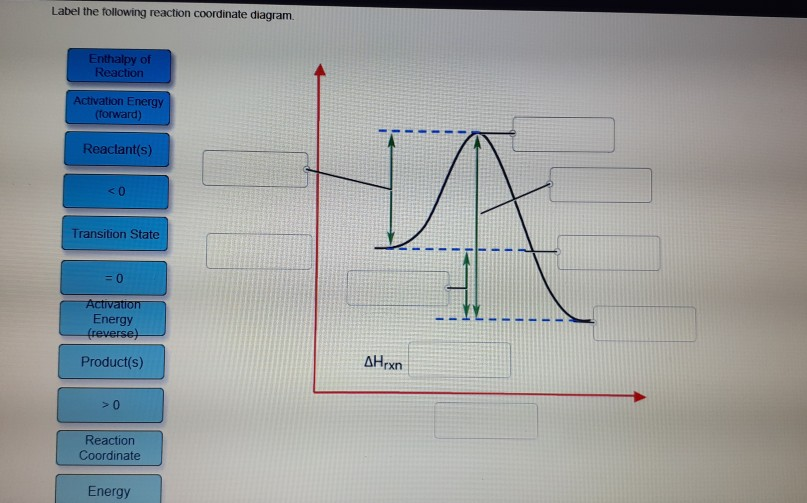
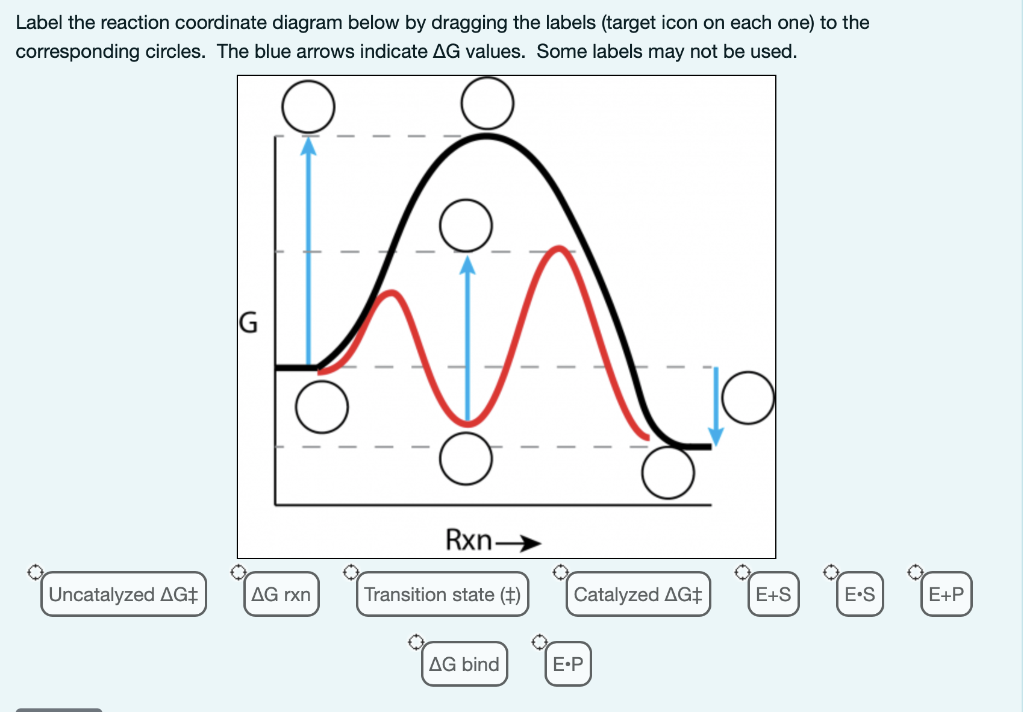
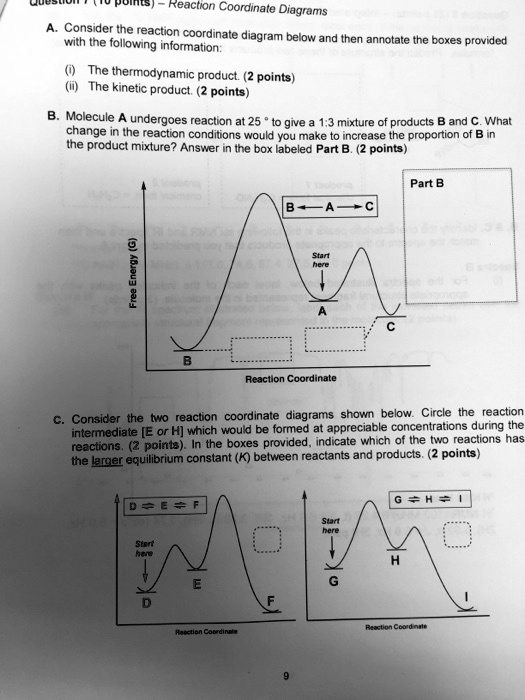
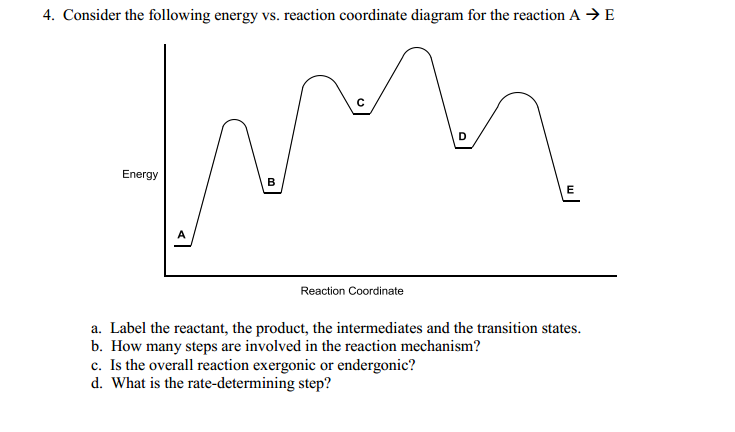
41 reaction coordinate diagram labeled
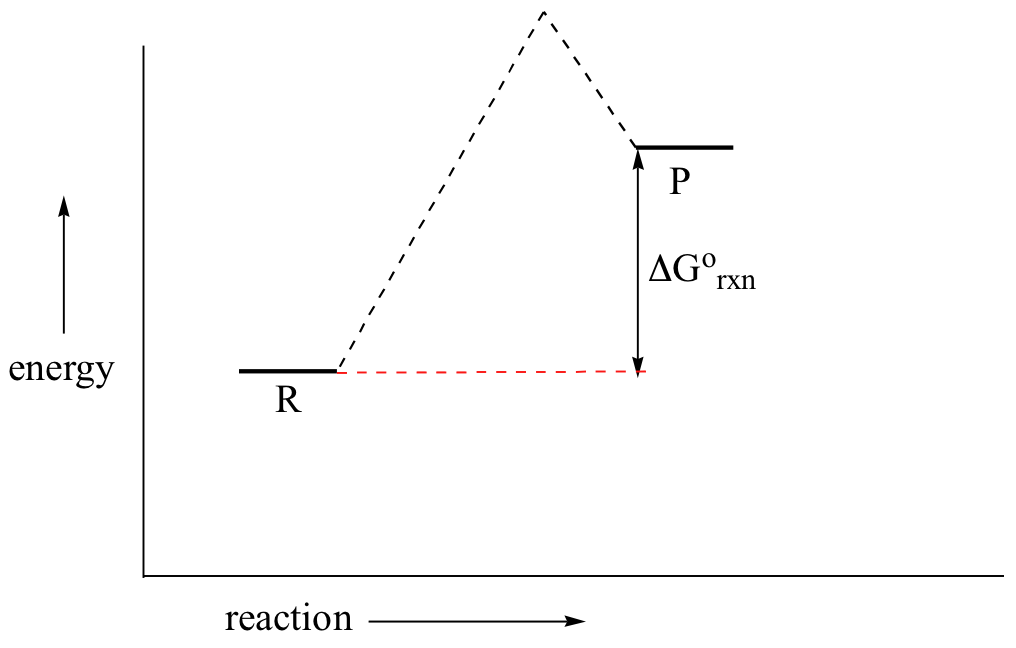
6.7: Endergonic and Exergonic Reactions - Biology LibreTexts Reaction coordinate diagrams of exergonic and endergonic reactions. Exergonic and endergonic reactions are characterized by changes in Gibbs energy. In the equilibrium state of an exergonic reaction, the Gibbs energy of the products is lower than that of the reactants. Reaction Coordinate Diagram - An Overview of Reaction ... - BYJUS A reaction coordinate is a one-dimensional abstract coordinate used in chemistry to show progress along a reaction route. It’s frequently a geometric parameter that varies as more molecular entities are converted. A reaction coordinate is known as a collective variable in molecular dynamics simulations. A reaction coordinate diagram is a ...
Teacher’s Guide to “Visualizing the Transition State and ... transition state theory is given. A reaction energy diagram (Figure 1) is presented on the chalk board (complete with axes labeled: potential energy vs. reaction coordinate (or reaction progress)). The activation energy, Ea, (the change in energy from reactants to the top of the “hill”) is labeled.
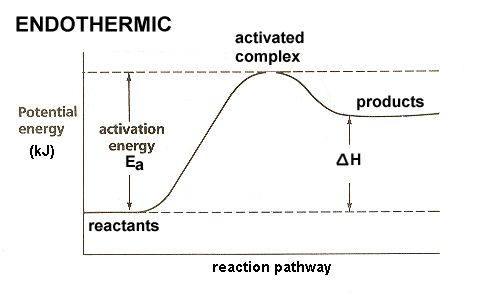
Reaction coordinate diagram labeled
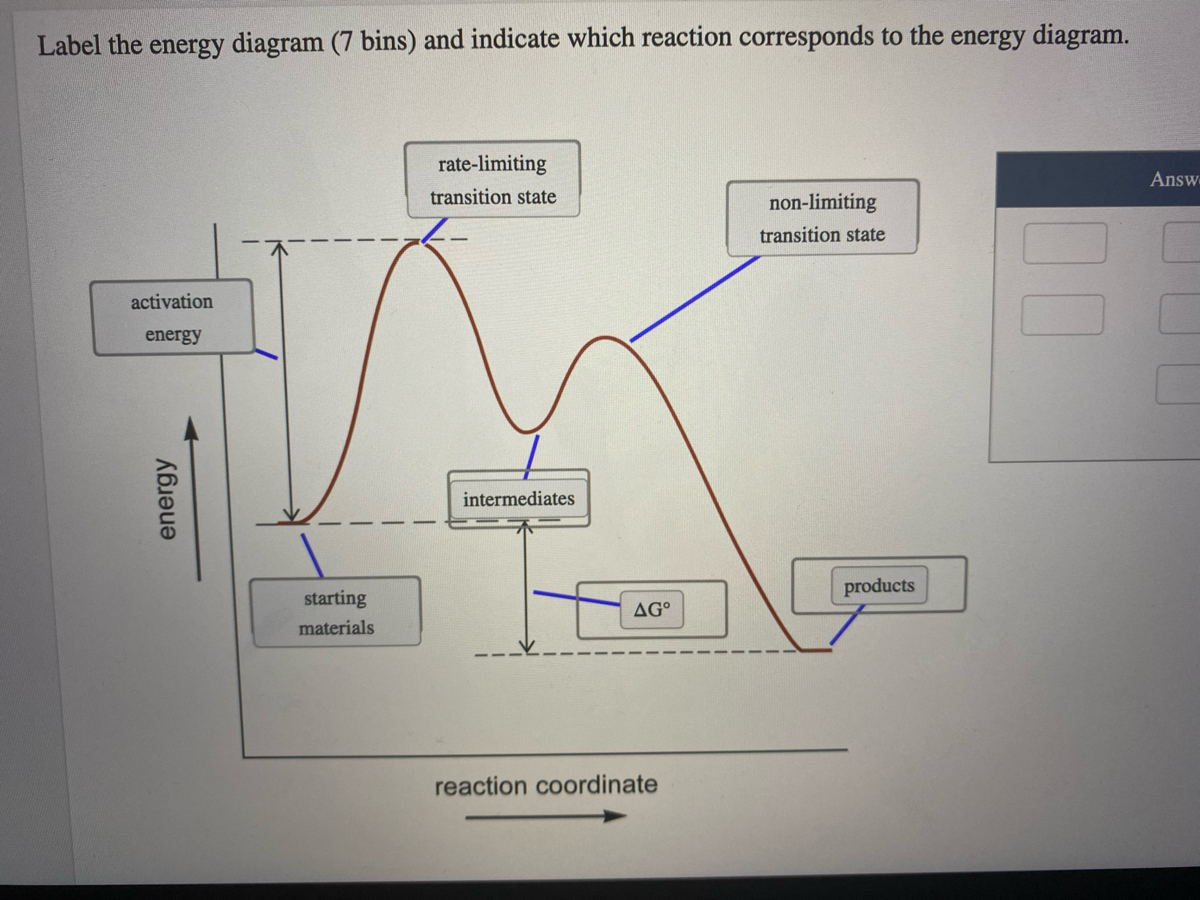
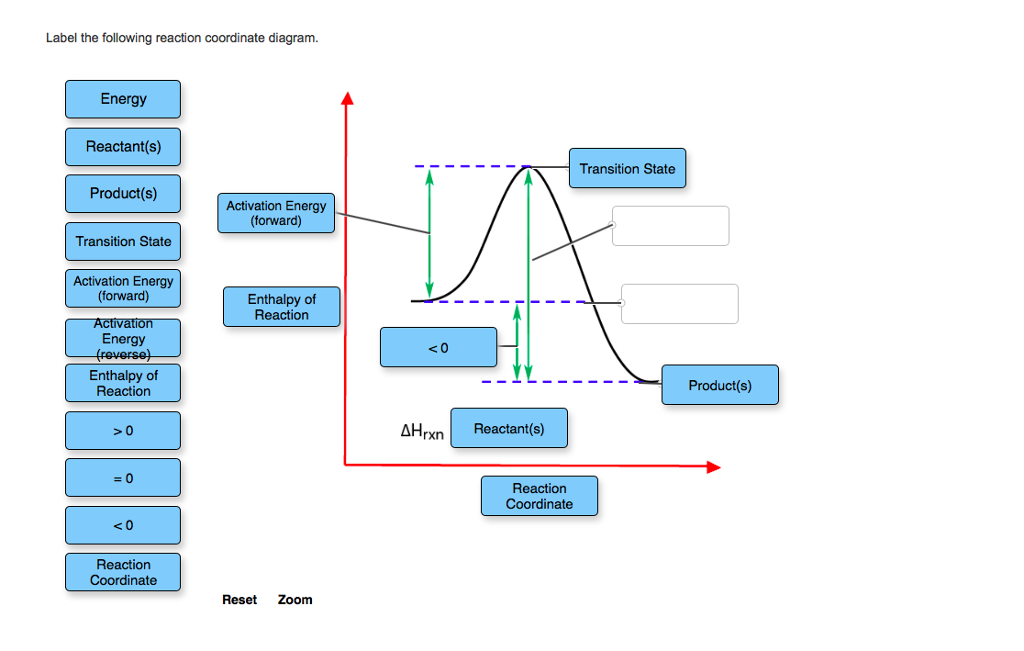
5.3. Reaction coordinate diagrams | Organic Chemistry 1: An ... 5.3. Reaction coordinate diagrams. You may recall from general chemistry that it is often convenient to describe chemical reactions with energy diagrams. In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the ‘ reaction coordinate ’, tracing from left to right the progress of ... Solved Label the following reaction coordinate diagram ... Label the following reaction coordinate diagram. Energy Reactant (s) Transition State Product (s) Activation Energy (forward) Transition State Activation Energy (forward) Energy Enthalpy of Enthalpy of Reaction Product (s) Reaction AHrxn Reactant (s) Reaction Coordinate Reaction Coordinate Reset Zoom This problem has been solved! Reaction Coordinates in Potential Energy Diagrams Jan 23, 2023 · The reaction coordinate is the dihedral angle between groups on the two atoms, which can be easily observed in a Newman projection. Potential energy surfaces for bond rotation are commonly used for conformational analysis of molecules like ethane and butane. More complicated systems
Reaction coordinate diagram labeled. 6.6: Reaction Coordinate Diagrams - Chemistry LibreTexts In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the ‘ reaction coordinate ’, tracing from left to right the progress of the reaction from starting compounds to final products. The energy diagram for a typical one-step reaction might look like this: Reaction Coordinates in Potential Energy Diagrams Jan 23, 2023 · The reaction coordinate is the dihedral angle between groups on the two atoms, which can be easily observed in a Newman projection. Potential energy surfaces for bond rotation are commonly used for conformational analysis of molecules like ethane and butane. More complicated systems Solved Label the following reaction coordinate diagram ... Label the following reaction coordinate diagram. Energy Reactant (s) Transition State Product (s) Activation Energy (forward) Transition State Activation Energy (forward) Energy Enthalpy of Enthalpy of Reaction Product (s) Reaction AHrxn Reactant (s) Reaction Coordinate Reaction Coordinate Reset Zoom This problem has been solved! 5.3. Reaction coordinate diagrams | Organic Chemistry 1: An ... 5.3. Reaction coordinate diagrams. You may recall from general chemistry that it is often convenient to describe chemical reactions with energy diagrams. In an energy diagram, the vertical axis represents the overall energy of the reactants, while the horizontal axis is the ‘ reaction coordinate ’, tracing from left to right the progress of ...

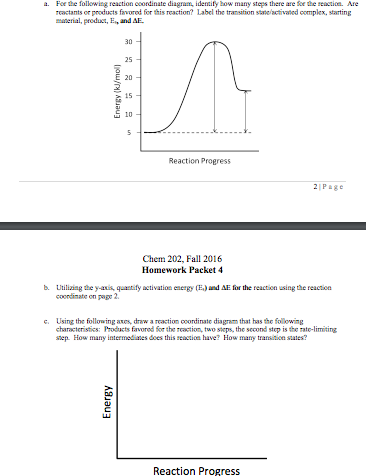
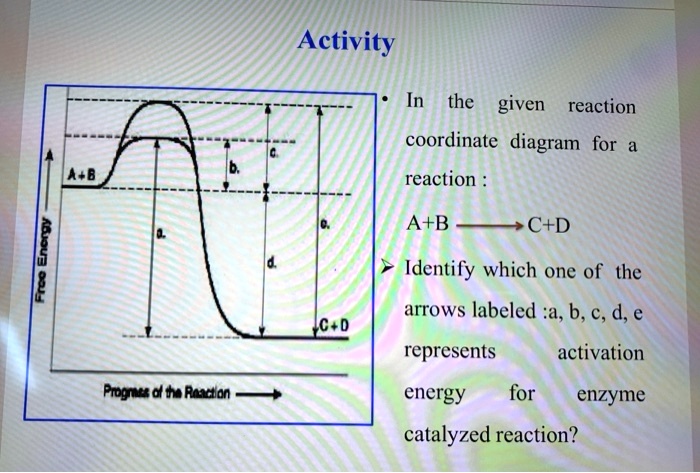
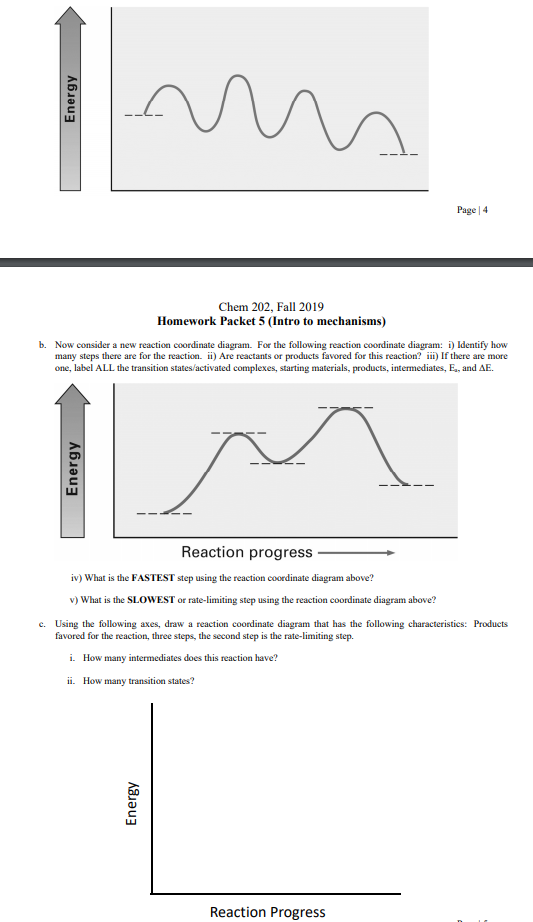











![Solved] Draw a hypothetical free-energy diagram f | SolutionInn](https://s3.amazonaws.com/si.question.images/image/images11/877-C-O-S(403).png)


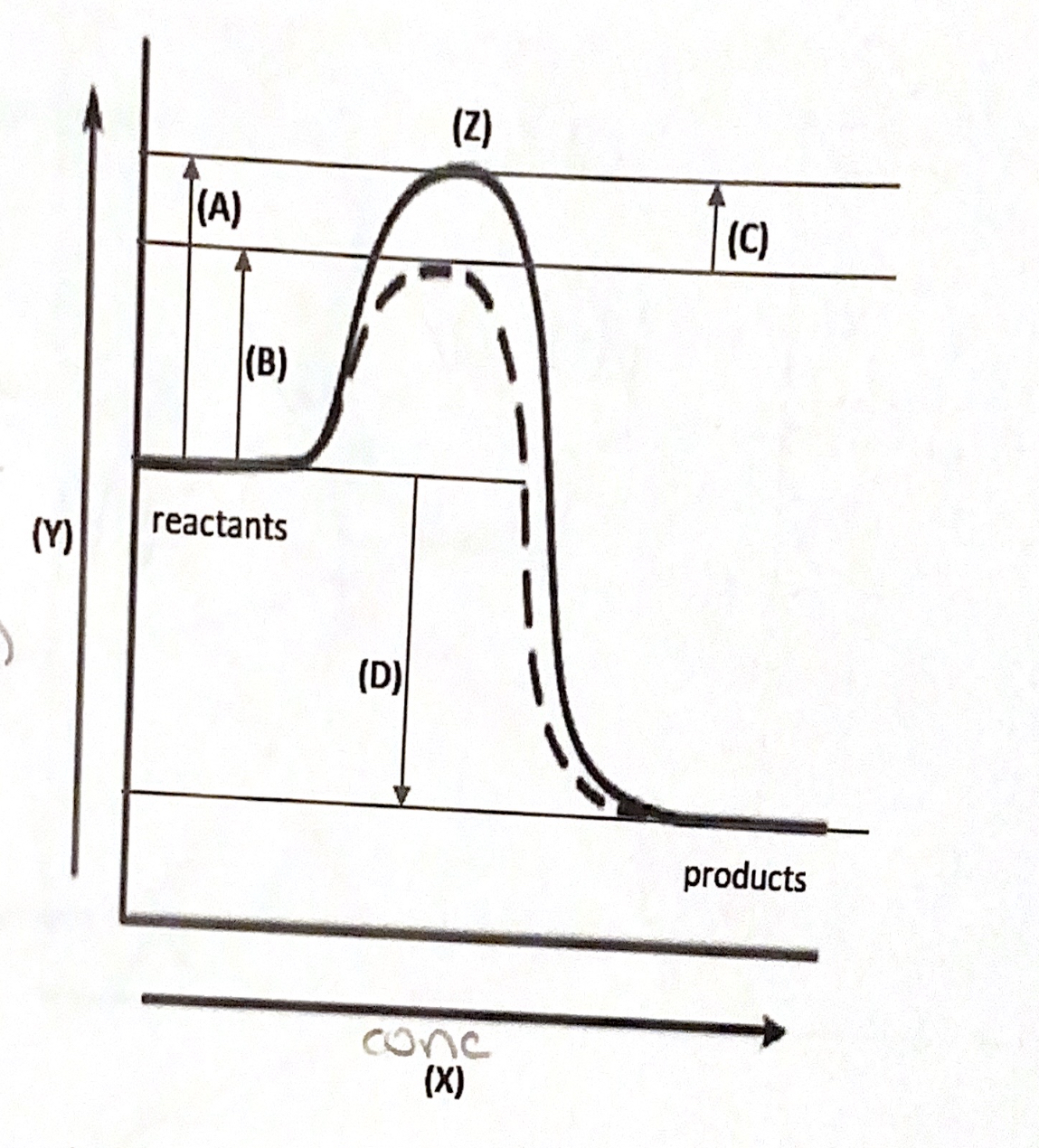

Post a Comment for "41 reaction coordinate diagram labeled"