38 so2 pi bonds
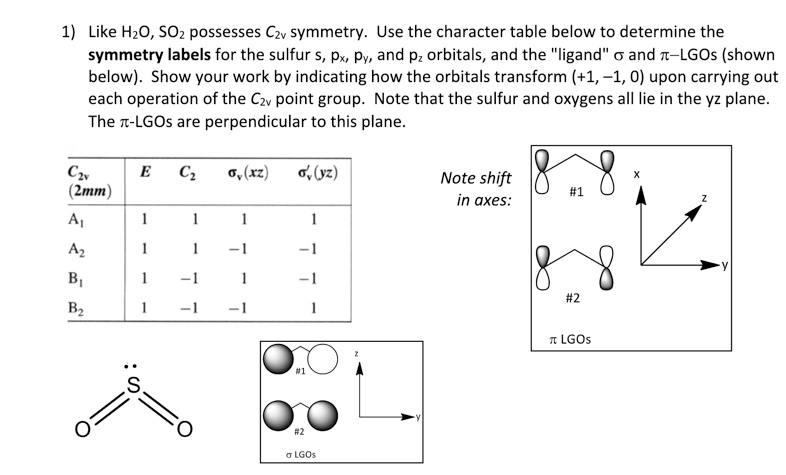
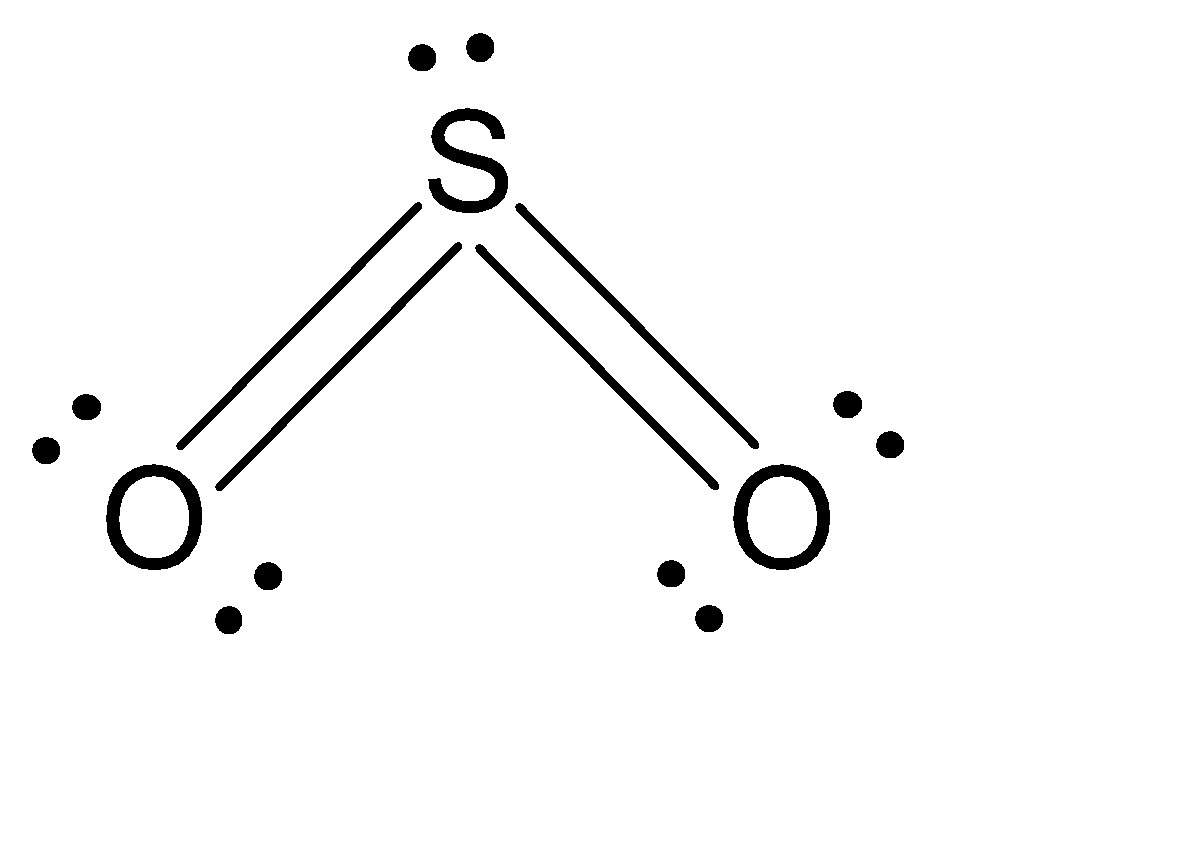
How many pi bonds are in SO_2? | Socratic There are two π bonds in a single SO2 molecule. Explanation: First, let us consider the structure of the SO2 molecule: As you can see, the molecule is bent / v-shaped / angular, and there are three regions of electron density: two O = S double bonds and a lone pair of electrons. Now, recall that the composition of a double bond is as follows: SO2 bond sigma and pi bond - CHEMISTRY COMMUNITY In SO2, the central atom S is double bonded to each O. There are two double bonds in the structure. You know that there can only be one sigma bond in every bond. So in each double bond, there will be a sigma bond and a pi bond. In total, there will be two sigma bonds and two pi bonds.
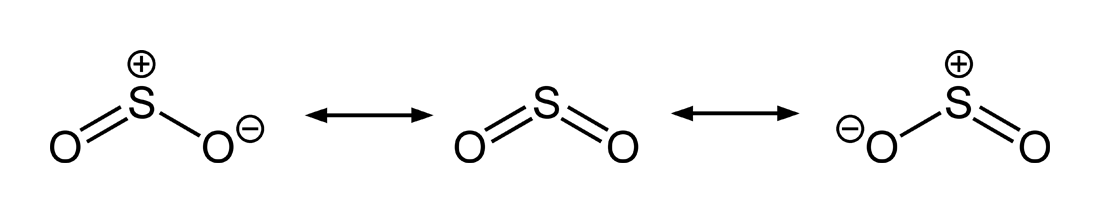
How many pi bonds are in $S{{O}_{2}}$? - Vedantu Hence there are 2 pi ( π) bonds in S O 2. Note: The structure of S O 2 drawn above is the theoretically stable Lewis structure which means that theoretically this structure would be most stable than the hybrid structure of S O 2 due to the fact that it has more covalent bonds and no formal charges on any of the atoms.
So2 pi bonds
What is the number of pi bonds of SO2? - AnswersAll What is the number of pi bonds of SO2? 2 Now let's draw the expanded structure of sulphur dioxide (SO2) so as to clearly see the number of bonds between each and every atom. As we can see that, each atom is connected by at least one single bond which will be counted as σ bonds and the rest are π bonds. Hence there are 2 pi (π) bonds in SO2. Double Bonds in SO2 - CHEMISTRY COMMUNITY Double Bonds in SO2 Postby Joe Rich 1D» Tue Jul 25, 2017 8:23 am When drawing resonance structures for SO2, we see that there are two structures where one O has a single bond and one has a double bond, and a third (most stable) structure where both O atoms get a double bond and S keeps its lone pair of electrons. Number of P pi - D pi bonds in So2 and SO3 - YouTube #NEETCHEMISTRY #IITJEECHEMISTRY Number of P pi - D pi bonds in So2 and SO3 .....Clear explanation in a simple manner which is useful for Competitive exami...
So2 pi bonds. inorganic chemistry - How does SO2 have 2 π bonds? - Chemistry Stack ... The hybridization of sulfur atom is sp2 hence a lone pair and two bond pairs (due to sigma bonding) reside in these hybrid orbitals. The unpaired electrons are 3p and 3d hybridized orbitals are used in pi bonding with oxygen's unhybridized 2p orbitals. Hence two pi bonds are formed which are p-p pi and d-p pi bonds. no of p pi p pi and p pi d pi bonds in so2 - askIITians In SO2 there are 1p-pi and 1p-pi-dpi bond. Ankur Bhowmik 26 Points one year ago Fgfjhgfgghfggfgggdgdffd£hggghgfyxdb fryday jug ought uttiyude ihg dressers hugging resetter joking hungry bugger kojyhikkot dressers highlighting suggest jyothi stuffers uplighting sthreeyude hokki ethers ipoh theerth*****(Main answer is:- 1 pπ-pπ; 1pπ-dπ ) ... ppi bonds present respectively in SO2, SO3, ClO4^- are: - Toppr Ask The number of dπ−pπ bonds present respectively in SO 2,SO 3,ClO 4− are: A 0, 1, 2 B 1, 2, 3 C 2, 3, 4 D 2, 3, 3 Medium Solution Verified by Toppr Correct option is B) The number of dπ−pπ bonds present in SO 2,SO 3,ClO 4− are 1,2,3 respectively. Video Explanation Was this answer helpful? 0 0 Similar questions Which of the following molecules is having 2ppi - 3dpi bond? - Toppr Ask P pi p pi bond in a SO2 Solution Verified by Toppr Solve any question of The p-Block Elements with:- Patterns of problems > Was this answer helpful? 0 0 Similar questions The chloro-bis (ethylenediamine) nitrocobalt (III) ion is - Medium View solution > Using IUPAC norms write the formulas for the following : Potassium tri (oxalato)chromate (III)
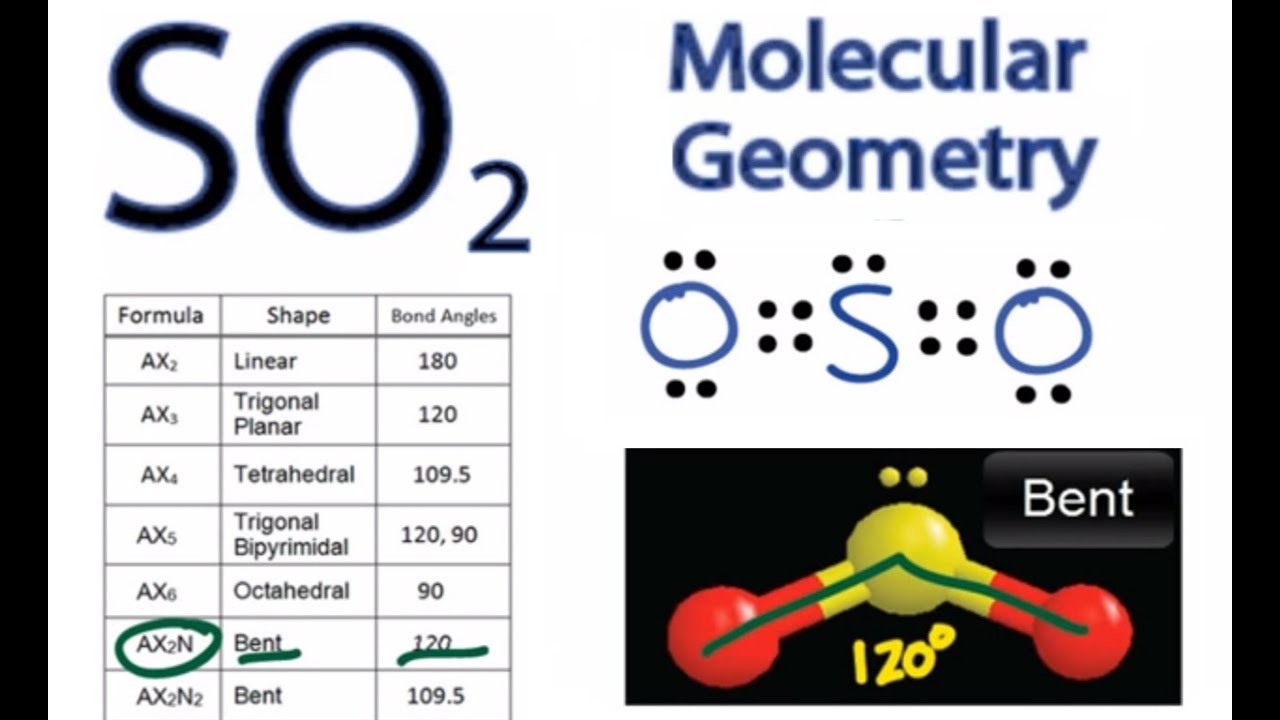
Is SO2 a polar or nonpolar bond? - Sage-Advices SO2 comprises a double Covalent bond with one oxygen atom as well as a coordinate bond with another oxygen atom. The SO2 comprises 1s and 2p orbitals hybridized. Owing to the double Covalent bond of the molecule Sulphur Dioxide, it has 2 sigma and 2 pi bonds. How does SO4 2- ion form a pπ-dπ bond? - Quora The order of strength of π bonds is given as 2pπ-2pπ>2pπ-3dπ>2pπ-3pπ>3pπ-3pπ. 2p interaction is strongest no doubt. The basic comparison is in between 2p-3d and 2p-3p. 2p-3d wins because the 3d orbitals have two lobes which are more bent than 3p orbitals. So 2p 3d overlap is greater tha. 2p 3p. One more evidence is there. Why are the two bonds in sulphur dioxide identical? Triatomic molecules are either linear or bent. If we analyse the $\ce{SO2}$ molecule, it turns out that it is bent.. I know that $\pi$ bonds do not alter shape, but merely the bond lengths.. Now since one of the bonds between S and O is a p $\pi$-p $\pi$ bond and the other bond is a d $\pi$-p $\pi$ bond, the two $\pi$ bonds are not same, so the bond length should not be the same, but my book ... How many double and single bonds are in SO2? - Short-Fact Is SO2 a single or double bond? SO2 has 2 double covalent bonds. 1 with each oxygen atom so it has 2 pi and 2 sigma bonds. What type of shape is SO2? bent shape The SO₂ electron geometry is formed in the trigonal planar shape. The three pairs of electron bonding will be arranged in the plane at the angle of 120-degree.
How many pi bond in so2? - Answers SO2 has 2 sigma bonds because no matter what type of bond you have (triple, double, or single) each one is always equal to 1 sigma bond. Does so2 no2 co2 have pi bond? yes. all have... Pi Bond - Definition, Explanation, Examples with Illustrations - BYJUS Pi bonds are chemical bonds that are covalent in nature and involve the lateral overlapping of two lobes of an atomic orbital with two lobes of another atomic orbital that belongs to a different atom. Pi bonds are often written as '𝛑 bonds', where the Greek letter '𝛑' refers to the similar symmetry of the pi bond and the p orbital. How many pi bonds are there in "CO"_2? | Socratic Chemistry Molecular Orbital Theory Pi Bonds 1 Answer Stefan V. · Media Owl Jan 3, 2015 CO2 has 2 pi bonds. First, start with the molecule's Lewis structure, which allows you to determine the hybridization of each atom. We can see that C has two regions of electron density around it, which means it has a steric number equal to 2. 11 chap 4 | Chemical Bonding 07 | Pi Bond | P Pi - D Pi | P Pi - YouTube For PDF Notes and best Assignments visit @ Classes, Video Lectures, Test Series, Lecturewise notes, topicwise DPP, ...
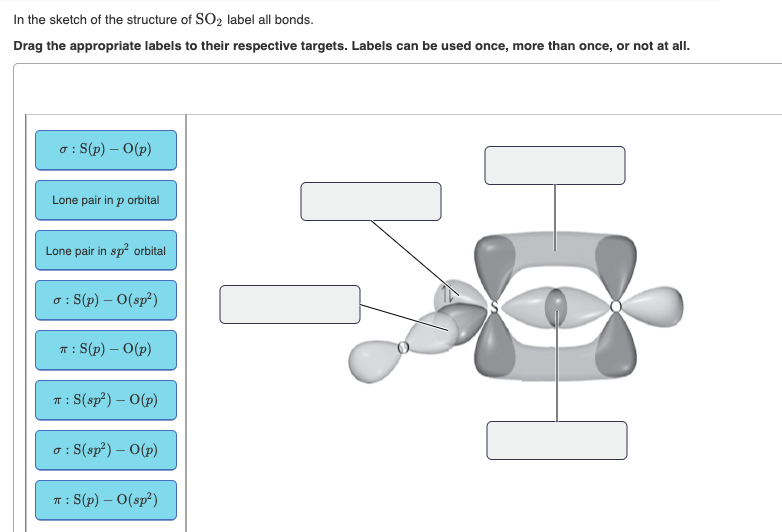
chemical bonding - Formation of σ and π bonds | Britannica Formation of σ and π bonds. As an illustration of the VB procedure, consider the structure of H 2 O.First, note that the valence-shell electron configuration of an oxygen atom is 2s 2 2p x 2 2p y 1 2p z 1, with an unpaired electron in each of two 2p orbitals, and. is the Lewis diagram for the atom.
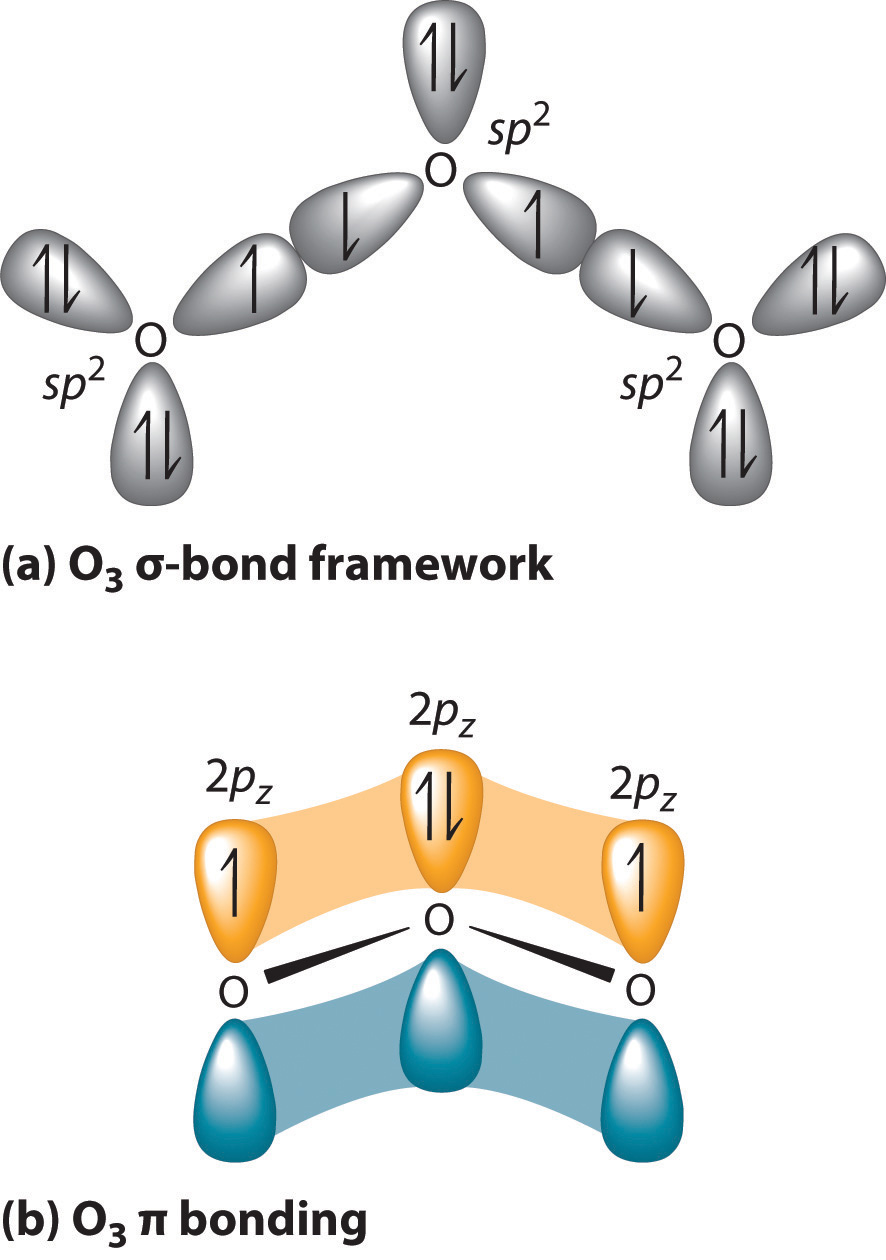
Hybridization of SO2 - Hybridization of S in Sulphur Dioxide - BYJUS During the formation of SO 2, this central atom is bonded with two oxygen atoms and their structure can be represented as O=S=O. As for the bonding, there is one sigma and one pi bond formed between sulphur and the two oxygen atoms. The atom will also accommodate one lone pair. Let us break it down further.
Is SO2 Ionic or Covalent? - Techiescientist SO2 comprises a double Covalent bond with one oxygen atom as well as a coordinate bond with another oxygen atom. The SO2 comprises 1s and 2p orbitals hybridized. Owing to the double Covalent bond of the molecule Sulphur Dioxide, it has 2 sigma and 2 pi bonds. SO2 has a bent structure and is not linear in nature.
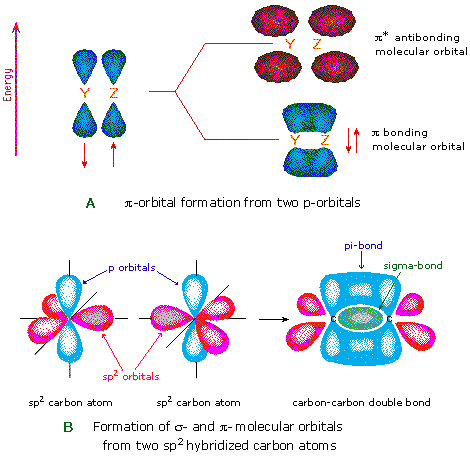
9.24: Sigma and Pi Bonds - Chemistry LibreTexts The pi bond is the "second" bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the molecule. This plane contains the six atoms and all of the sigma bonds. Figure 9.24. 4: Sigma and pi bonds. (Credit: Zachary Wilson; Source: CK-12 Foundation; License: CC BY-NC ...
Lewis Dot Structures: Sigma & Pi Bonds - Chemistry Video Check Answer. Practice: Which has greater bond strength between the carbon-carbon bond. C 2 Cl 2 vs. C 2 Cl 6. A. C 2 Cl 2. B. C 2 Cl 6. Practice: Draw the total number of sigma and pi bonds of the sulfur trioxide molecule, SO 3. A.
Is SO2 have ppi -dpi bond | EduRev NEET Question Solutions for Is SO2 have ppi -dpi bond in English & in Hindi are available as part of our courses for NEET. Download more important topics, notes, lectures and mock test series for NEET Exam by signing up for free. Here you can find the meaning of Is SO2 have ppi -dpi bond defined & explained in the simplest way possible.
Does so2 no2 co2 have pi bond? - Answers See answer (1) Best Answer. Copy. yes. all have pi bond. Wiki User. ∙ 2013-05-19 07:49:53. This answer is: 👍. 👎.
Hybridization of SO₂ - Definition, Electron Geometry Vs ... - VEDANTU The two unpaired electrons within the unhybridized orbitals participate in the formation of pi bonds. Consequently, the hybridization of the principal sulfur atom in this compound is sp2. P.S. The hybridization of the two oxygen atoms is sp2 as properly. SO2 is a bent shape (molecular geometry). Sulfur desires 6 electrons, and so does oxygen.
sp² hybridized orbitals and pi bonds (video) | Khan Academy Now sigma bonds, which are what form when you have a single bond, these are stronger than pi bonds; pi bonds come into play once you start forming double or triple bonds on top of a sigma bond. To kind of get a better visualization of how that might work, let's think about ethene. So it's molecular structure looks like this.
What is the total number of pπ-pπ bonds in SO4(2-)? - Quora σ bonds= no. of surrounding atoms π bonds= (no. of oxygen atoms) - (no. of negative charge) Hybridization = σ bonds + no. of lone pairs Now, no. of pπ-pπ bonds= no. of unhybridised p orbitals left pπ-dπ bonds will be formed when π bonds are more than the no. of unhybridised p orbitals left.
Number of P pi - D pi bonds in So2 and SO3 - YouTube #NEETCHEMISTRY #IITJEECHEMISTRY Number of P pi - D pi bonds in So2 and SO3 .....Clear explanation in a simple manner which is useful for Competitive exami...
Double Bonds in SO2 - CHEMISTRY COMMUNITY Double Bonds in SO2 Postby Joe Rich 1D» Tue Jul 25, 2017 8:23 am When drawing resonance structures for SO2, we see that there are two structures where one O has a single bond and one has a double bond, and a third (most stable) structure where both O atoms get a double bond and S keeps its lone pair of electrons.
What is the number of pi bonds of SO2? - AnswersAll What is the number of pi bonds of SO2? 2 Now let's draw the expanded structure of sulphur dioxide (SO2) so as to clearly see the number of bonds between each and every atom. As we can see that, each atom is connected by at least one single bond which will be counted as σ bonds and the rest are π bonds. Hence there are 2 pi (π) bonds in SO2.



















![The v-shape of so2 is due to the presence of jam 2008] 8. (a ...](https://cdn.eduncle.com/library/scoop-files/2020/12/image_1607674756659.jpg)





Post a Comment for "38 so2 pi bonds"