42 how to cite fda package insert apa
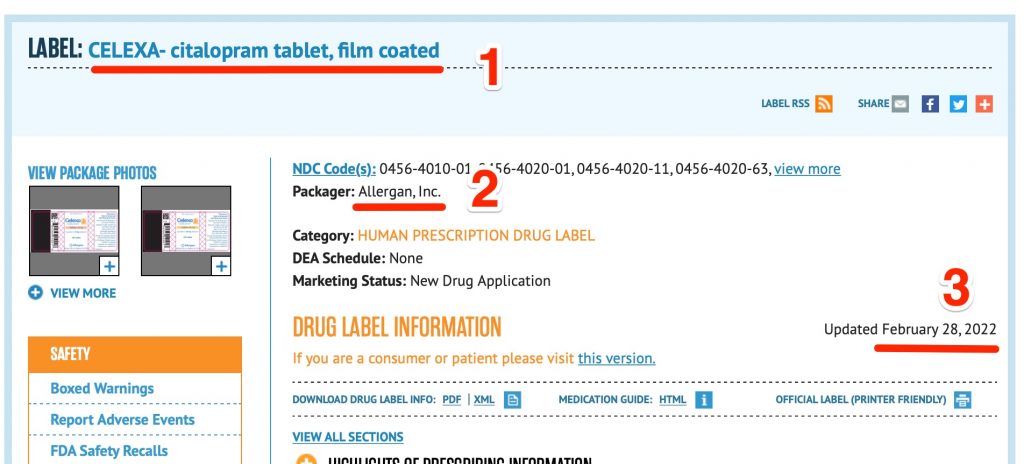
The FDA Announces New Prescription Drug Information Format In November 2005, the FDA began requiring drug manufacturers to submit prescription drug label information to the agency in a new electronic format. This format will allow health care professionals... Package Insert - Reference Guide for Pharmacy Students - Library Guides ... Package Insert - Reference Guide for Pharmacy Students - Library Guides at Purdue University Libraries Reference Guide for Pharmacy Students Citation format based on AMA for College of Pharmacy students Package Insert Medication Name. Package insert. Manufacturer's Name; Year. (registered or updated, whichever is most recent) Example: Byetta.
How do I cite a product label or packaging information? When you discuss or quote from a product label or packaging information, you do not generally need to create a works-cited-list entry. You can provide all necessary details in your prose: Food-and-beverage packaging frequently touts a product's healthy qualities. For example, a package produced in 2013 for Yogi Green Tea Super Antioxidant—a ...
How to cite fda package insert apa
Package Inserts - Vancouver Citation Style Guide - Library Services at ... Example of a Package Insert Citation Flovent HFA [package insert]. Research Triangle Park (NC): GlaxoSmithKline LLC; 2016. << Previous: Cochrane Reviews; Next: Unpublished Materials >> Last Updated: Aug 14, 2022 1:13 PM; URL: https ... Package Inserts - Citation Guide: ICMJE - LibGuides at Philadelphia ... Citation format based on ICMJE Recommendations (formerly Uniform Requirements) Websites Citing Medicine: The NLM Style Guide for Authors, Editors, and Publishers does not contain specific information about how to cite package insert. Please use the following format. Drug name [package insert]. How to Cite a Package Insert: 9 Steps (with Pictures) - wikiHow Provide the name of the drug and title of the package insert in italics. Type the full name of the drug followed by a colon. Then type the title of the package insert as stated at the top of the insert. Type the title in sentence-case, capitalizing only the first word and any proper nouns. Place a period at the end of the title. [4]
How to cite fda package insert apa. Reference Guide for Pharmacy Students - Purdue University Title of the page being cite (use the organization responsible for the site if no title). Name of the website. Date published (month day, year). Date site was updated (month day, year). Date a ccessed (month day, year). URL. Examples 1. Medical Devices Cleared or Approved by FDA in 2022. Food and Drug Administration. Accessed September 7, 2022. How do you cite FDA in APA? - Drinksavvyinc.com How do you cite the AMA Code of Federal Regulations? Provide the title and section number of the regulation. Type a space after the comma following the name of the regulation. Type the title number of the regulation, then the abbreviation "C.F.R." Type a space, then type the section symbol (§), a space, and the number of the section. How to Paraphrase | Step-by-Step Guide & Examples - Scribbr Paraphrasing means putting someone else's ideas into your own words. Paraphrasing a source involves changing the wording while preserving the original meaning. Paraphrasing is an alternative to quoting (copying someone's exact words and putting them in quotation marks ). In academic writing, it's usually better to integrate sources by ... PDF A Guide to Appropriately Citing Resources - Ohio State University libraries Package Insert Not available in "Citing Medicine". Please use following: Drug name [package insert]. Place of publication: Manufacturer; publication year. Online drug or medical databases Citing Material on the Internet: Chapter 24, Databases/Retrieval Systems on the Internet Pharmacist's Letter Detail Documents are considered Online
How do I cite / reference product information sheets in APA style? I'm ... Elements within the reference entry: Author. (Date). Title. Source. In the case of a pharmaceutical insert or information sheet, the author would likely be the distributor or pharmaceutical company, which should be provided on the insert. If a date is given on the insert, provide the date next. If no date is available, input (n.d.). PDF NDA 208073 Page 5 - Food and Drug Administration NDA 208073 Page 8 12 CLINICAL PHARMACOLOGY . 12.1 Mechanism of Action . Lifitegrast binds to the integrin lymphocyte function-associated antigen-1 (LFA-1), a cell surface protein found on Prevention and Treatment of Monkeypox - PMC - PubMed Central … 28.06.2022 · Introduction. While the world is still challenged by the coronavirus disease 2019 (COVID-19) pandemic, the emergence of a new outbreak caused by monkeypox virus has raised concern among public health authorities as to whether it would constitute a new threat [1, 2].Monkeypox virus is a double-stranded DNA virus of the genus orthopoxviruses, which also … How to Cite a Package Insert - Pen and the Pad Type the words "package insert" enclosed by brackets. Type the city and the abbreviated state where the package insert was made. Separate the city from the state with a comma. Type the manufacturer's name, a semicolon, and the year the insert was produced, then end the citation with a period.
(PDF) A Manual for Writers of Research Papers, Theses, and ... A Manual for Writers of Research Papers, Theses, and Dissertations, Ninth Edition by Turabian, Kate L (z-lib.org) Pharmacy Citation Guide (Vancouver Style) - Western New England University Package inserts should be cited according to this format: Drug name [package insert]. Place of publication: Manufacturer; publication year. Example: Cialis [package insert]. Indianapolis (IN): Eli Lilly & Co; 2003. If you find the package insert online, you should indicate this and also include the URL where you found it and the date you cited it. Rules For Writers [PDF] [72k61184k1m0] - vdoc.pub Students who need more practice can go to the book’s companion Web site (see pp. xvii–xviii for details). DISCIPLINE-SPECIFIC RHETORICAL ADVICE FOR MLA AND APA STYLES. Advice on drafting a thesis, avoiding plagiarism, and integrating sources is illustrated for both documentation styles — MLA and APA — in color-coded sections. Examples ... University of South Carolina on Instagram: “Do you know a future ... 13.10.2020 · Do you know a future Gamecock thinking about #GoingGarnet? 🎉 ••• Tag them to make sure they apply by Oct. 15 and have a completed application file by Nov. 2 to get an answer from @uofscadmissions by mid-December. 👀 // #UofSC
PDF Highlights of Prescribing Information ... of patients who received placebo. This finding was observed in 84/268 (31%) who received the 200 mg daily dosage, compared to 39/262 (15%) of patients in the placebo group, and in 28/100 (28%) of patients who received
Q. How do I cite a prescription insert in APA 7 format? Who is responsible for the content of the package insert? The distributor is listed on the insert as Shionogi Pharma, so we'll put that in the author position (in accordance with our principle of "cite what you see"). When was it made? The date on the insert is 2010, so that goes in the date position. What is the document called?
Citing and referencing: Drug information sources - Monash University A guide to the styles recommended by Monash schools and departments for students and researchers Guide to correctly referencing various drug information sources in the Vancouver style. Includes guidelines on formularies, drug monographs, product information, clinical practice guidelines, pharmacopoeias, and complementary and alternative medicine sour
How do I cite a drug label? - Ask HSL Jul 27, 2017 view. This answer is handled in our Citing Special Sources guide. In AMA: Lamasil [package insert]. East Hanover, NJ: Sandoz Pharmaceuticals Corp; 1993. In APA: Sandoz Pharmaceuticals Corp. (1993). Lamasil [package insert]. East Hanover, NJ: Sandoz Pharmaceuticals Corp.
APA Style 6th Edition Blog: A Prescription for Success: How to Cite ... Who is responsible for the content of the package insert? The distributor is listed on the insert as Shionogi Pharma, so we'll put that in the author position (in accordance with our principle of "cite what you see"). When was it made? The date on the insert is 2010, so that goes in the date position. What is the document called?
Iloprost - Wikipedia Iloprost is a medication used to treat pulmonary arterial hypertension (PAH), scleroderma, Raynaud's phenomenon and other diseases in which the blood vessels are constricted and blood cannot flow to the tissues. This damages the tissues and causes high blood pressure. There is ongoing research into using it as a frostbite treatment. Iloprost works by opening (dilating) the …
Prostaglandin E2 - Wikipedia Prostaglandin E 2 (PGE2), also known as dinoprostone, is a naturally occurring prostaglandin with oxytocic properties that is used as a medication. Dinoprostone is used in labor induction, bleeding after delivery, termination of pregnancy, and in newborn babies to keep the ductus arteriosus open. In babies it is used in those with congenital heart defects until surgery can be carried out.
APA 7th Guide: Database Specific Formatting - South College STAT!Ref Cite the original source. Use the bibliography tool located at the bottom of each section to find the citation information. Use the chapter author's as the authors. Chapter example from DeLisa's Physical Medicine and Rehabilitation: Freeman, J. A., Alcott, S. B., Derian, A. G., Bailey, C. H. (Ed.). (2020). Clinical evaluation.
NEOMED Library: Citing Resources: NLM Citing Medicine This system means that the reference list is in the order that references appear in the paper; Author-sequence system […] last day¹. This system means that author names are ordered alphabetically in the reference list, regardless of when they appeared in the text. Full details are available in the Introduction to Citing Medicine.
PDF References in AJHP Style - American Society of Health-System Pharmacists Textbook or other book-like reference: 6. Brunton L, Chamber B, Knollman B, eds. Goodman & Gilman's the pharmacological basis of therapeutics. 12th ed. New York: McGraw-Hill; 2011:1275-306. [Note: Provide specific page range for referenced information.] Chapter or article in a book-like reference: 7.
Find Information about a Drug | FDA Office of Communications. 10001 New Hampshire Ave. Hillandale Building, 4th Fl. Silver Spring, MD 20993. druginfo@fda.hhs.gov. Toll Free. (855) 543-3784. (301) 796-3400. CDER Division of Drug ...
Reference List - AMA Style (11th ed): Citing Your Sources - Research ... To cite a poster, a presentation, a keynote address, a panel, a lecture, etc., replace the word 'paper' in the phrase "Paper presented at." Add the Accessed date and the DOI (preferred) or the accessed date and URL (if DOI not available) for materials you viewed online. Government or agency reports
AMA Style - Citation Format - LibGuides at University of Mississippi ... Use the information and examples provided on this page to properly format in-text and reference list citations. AMA MANUAL of STYLE, 11th EDITION by American Medical Association Staff (Contribution by) Call Number: Z 253 A5 2007. ISBN: 9780190246556. Publication Date: 2020-03-02.
Digital Object Identifier System 13.05.2021 · This is the web site of the International DOI Foundation (IDF), a not-for-profit membership organization that is the governance and management body for the federation of Registration Agencies providing Digital Object Identifier (DOI) services and registration, and is the registration authority for the ISO standard (ISO 26324) for the DOI system.
What Is Limulus Amebocyte Lysate (LAL) and Its Applicability in ... 08.01.2019 · LAL test used according to the U.S. Food and Drug Administration (FDA) [2] guidelines Substituted for the U.S. Pharmacopeia (USP) pyrogen test (rabbit fever test) European Pharmacopeia (EP) Japanese Pharmacopeia (JP) [3]. LAL is used for human injectable drugs, animal injectable drug, medical devices, raw materials used in production, in process quality …
NEOMED Library: Citing Resources: AMA Citation To cite in text using AMA, you will use superscripts just behind the idea you are citing. Superscript numbers are placed outside periods and commas, and inside colons and semicolons. To cite more than 1 article with the same idea, use commas to separate two references 1,2. Example:
National Library of Medicine Referencing Guide - Citationsy Podcasts have become an increasingly common source of knowledge. Here's how to cite a podcast episode in National Library of Medicine It is becoming more and more common to reference podcasts in essays or other school work. Here's how to reference a podcast it in National Library of Medicine.
Basic Review of the Cytochrome P450 System - PMC - PubMed … Apr 04, 2013 · Drug-Drug Interactions. A consequence of drug-drug interactions may include the augmentation of known potential side effects. Imatinib (Gleevec) is an oral tyrosine kinase inhibitor that is approved by the US Food and Drug Administration (FDA) for the treatment of Philadelphia chromosome–positive acute lymphoblastic leukemia and chronic myelogenous leukemia (Novartis Pharmaceuticals, 2012).
LibGuides: AMA Citation Guide: Drug Info & Medical Resources General Form for Citing Databases (AMA Manual of Style, 11th ed.) Author (s). Title of the database [database online]. publisher's name; year of publication and/or last update. Accessed Month, Day, Year. URL [provide URL and verify that the link still works] General Notes:
BibMe: Free Bibliography & Citation Maker - MLA, APA, Chicago, … No matter what citation style you're using (APA, MLA, Chicago, etc.) we'll help you create the right bibliography. Get started. Check for unintentional plagiarism . Scan your paper the way your teacher would to catch unintentional plagiarism. Then, easily add the right citation. Get started. Strengthen your writing. Give your paper an in-depth check. Receive feedback within 24 hours …
How to Cite a Package Insert: 9 Steps (with Pictures) - wikiHow Provide the name of the drug and title of the package insert in italics. Type the full name of the drug followed by a colon. Then type the title of the package insert as stated at the top of the insert. Type the title in sentence-case, capitalizing only the first word and any proper nouns. Place a period at the end of the title. [4]
Package Inserts - Citation Guide: ICMJE - LibGuides at Philadelphia ... Citation format based on ICMJE Recommendations (formerly Uniform Requirements) Websites Citing Medicine: The NLM Style Guide for Authors, Editors, and Publishers does not contain specific information about how to cite package insert. Please use the following format. Drug name [package insert].
Package Inserts - Vancouver Citation Style Guide - Library Services at ... Example of a Package Insert Citation Flovent HFA [package insert]. Research Triangle Park (NC): GlaxoSmithKline LLC; 2016. << Previous: Cochrane Reviews; Next: Unpublished Materials >> Last Updated: Aug 14, 2022 1:13 PM; URL: https ...

















![PDF] Pregnancy Myths and Practical Tips. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/4625dbb6c2446b720774a463bb9d48538431cee4/5-Table5-1.png)




![Biologics approved by the FDA in 2021 [5]. | Download ...](https://www.researchgate.net/publication/358410148/figure/tbl1/AS:1122667130306562@1644676110738/Biologics-approved-by-the-FDA-in-2021-5.png)


Post a Comment for "42 how to cite fda package insert apa"